Native lipid environment reveals more about peptide transporters
Peptide transporters are membrane proteins with a remarkable substrate promiscuity which makes them important targets for drug delivery. CSSB scientist Christian Löw’s (EMBL) group in collaboration with Dr. Hagen Hofmann’s group from the Weizmann Institute of Science in Israel have recently demonstrated that placing these peptide transporters in a more native lipid environment reveals additional structural and functional details. Their results, published in the international edition of Angewandte Chemie, will help further the development of an atomic level understanding of the transport cycle.
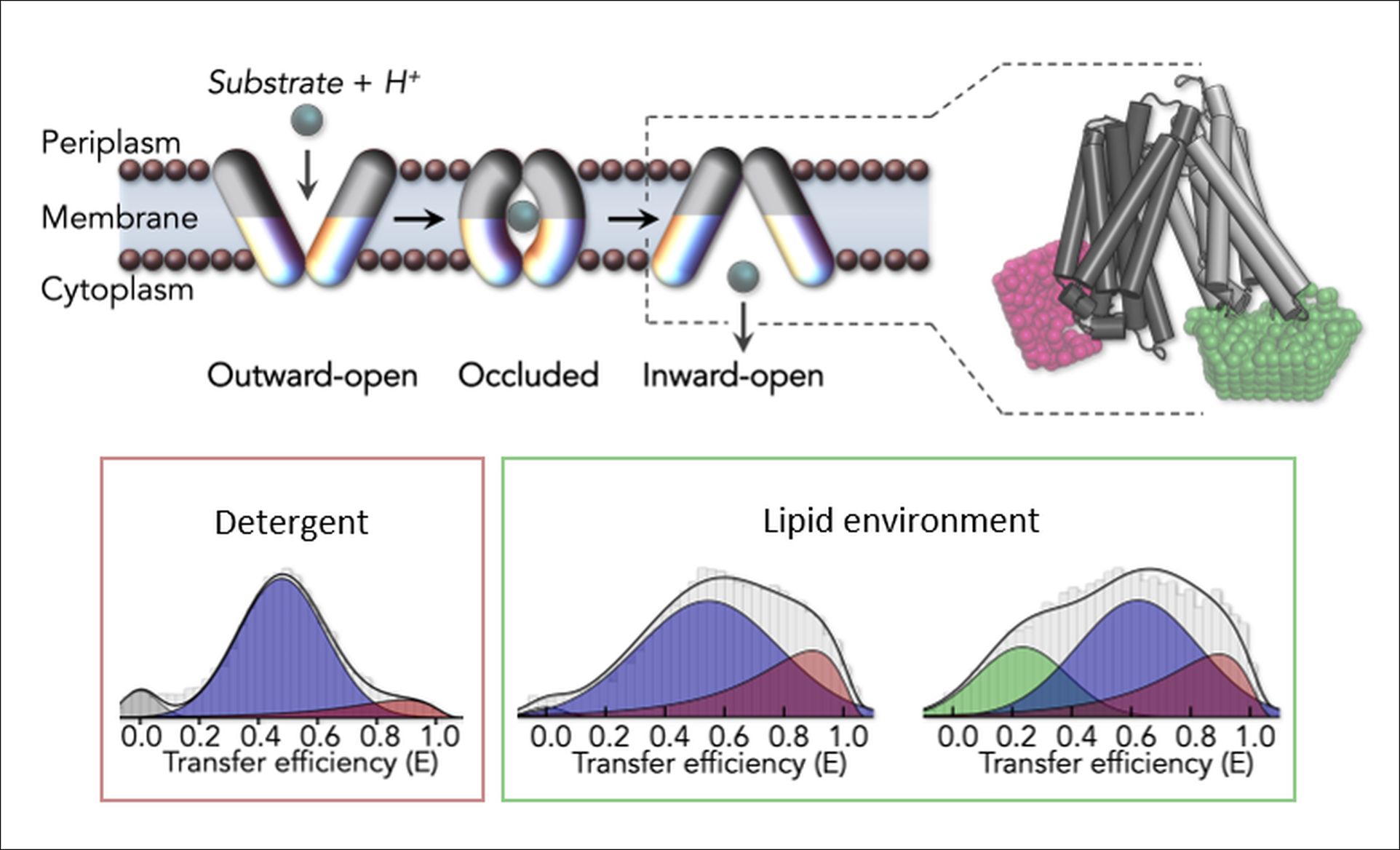
To determine their molecular structure, membrane proteins are typically extracted from their native environment using detergents and then crystallized for examination via x-ray crystallography. The strongest limitation of this approach is that it remains unclear whether the structural and functional integrity of the protein is compromised when it is removed from its native environment. “To better understand the impact of detergents on peptide transporters, we decided to conduct an in-depth comparison of their conformations in detergent and lipid environments,” explains Löw.
The scientists used single-molecule Förster resonance energy transfer (smFRET), a technique which uses fluorescent dyes to measure the distances between these probes, to examine DtpA a member of the peptide transporter subfamily known as Proton-dependent oligopeptide transporter or POTs. The smFRET measurements of DtpA in a detergent environment revealed that it is trapped in a single inward-open conformational state. “However, when DtpA was placed in a lipid disc, we observed a restored adaptability allowing for different states including the outward-open state and a newly identified extreme inward-open conformation,” explains Kim Bartels one of the papers first authors.
The results indicate that DtpA is not only dynamic and flexible but also responds sensitively to its lipid environment. “We have demonstrated that peptide transporter’s lipid environment plays a critical role in its functionality,” explains Löw “thus underscoring the importance of developing techniques for examining membrane proteins in their native lipid environment.” The Löw and Hofmann group will use the techniques developed during this study to explore the complete transport cycle of peptide transporters in their lipid environment on a single molecule level in real time.
Reference:
Lasitza-Male T, Bartels K, Jungwirth J, Wiggers F, Rosenblum G, Hofmann H, Löw C. (2020) Membrane Chemistry Tunes the Structure of a Peptide Transporter. Angew Chem Int Ed Engl. doi: 10.1002/anie.202008226