Control Mechanism of Vesicle Constriction Understood
MHH Researchers solve the structure of an essential building block of the dynamin helix. Their findings are published in the scientific journal “Nature.”
The regulated formation and release of vesicles from cellular membranes is an essental process for eukaryotic organisms. For example, the communication between nerve cells is critically dependent on the process. The protein dynamin is essential for the formation of many vesicles. Scientists from the Institute for Biophysical Chemistry at Hannover Medical School (MHH) together with colleagues from the Max Delbrück Center in Berlin and Freie Universität Berlin have discovered how dynamin uses a regulated process to tie itself into a screw-like structure. Moreover, they demonstrated how specific mutations impair the function of dynamin, for example, mutations occurring in the congenital muscle disorders Charcot–Marie–Tooth neuropathy and Centronuclear Myopathy. This is an important initial step in the development of new therapeutic approaches. The scientists have published their findings in the scientific journal “Nature.”
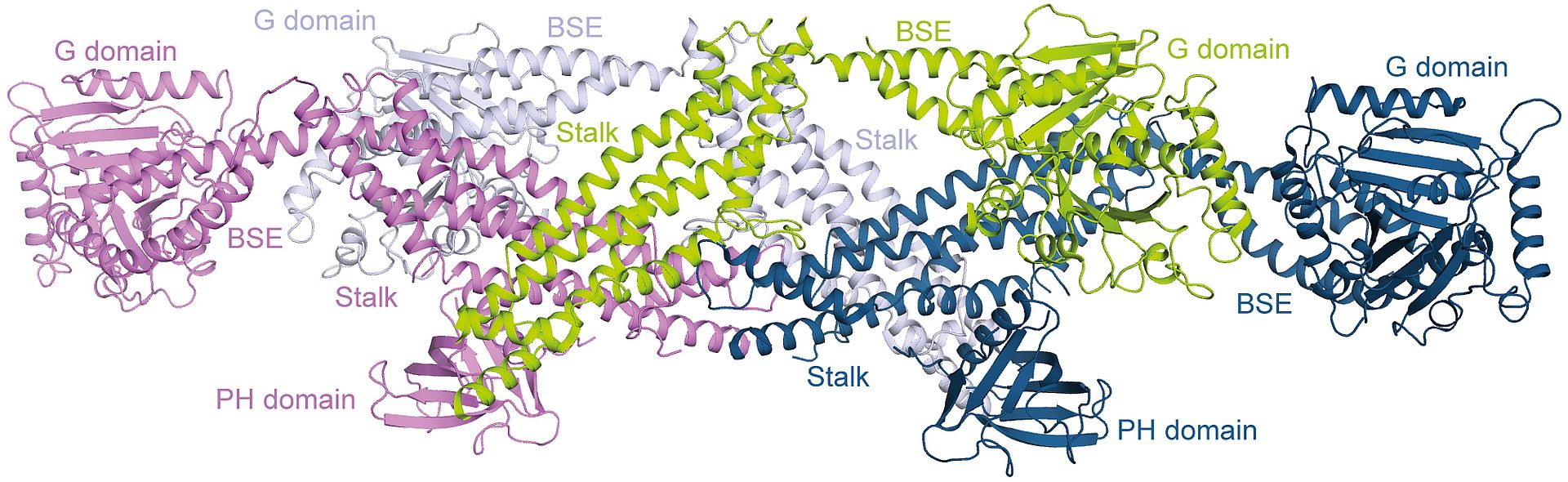
Nerves guide signals, which are packed as messenger substances in vesicles, from one nerve cell to the next. These vesicles are formed through membrane protuberances, which are severed by dynamin: without consuming any energy, a chain of dynamin wraps itself multiple times around the neck of the budding vesicle. In a second, energy-dependent step, the dynamin helix is constricted.
The scientists can now demonstrate that the building blocks of the helix are comprised of four dynamin molecules, called dynamin tetramers. Additionally, based on an accuracy of approximately a half a millionth of a millimetre, the scientists could determine how the first sub-step of the building of the dynamin helix is regulated. “The dynamin tetramers possess exactly the right curvature for the building of the helix and the helix can be built without further energy use through a simple side-by-side placement of the tetramers,” explains Dr Susanne Eschenburg, MHH Institute for Biophysical Chemistry. “The contact points between the tetramers are blocked until the correct start signal comes from the cell. Only then can the helix be built,” adds her colleague Dr Thomas Reubold.
For Additional Information please contact Dr. Susanne Eschenburg, Institute for Biophysical Chemistry. Telephone (0511) 532-8655, eschenburg.susanne@mh-hannover.de.
Source: Hannover Medical School (MHH)
Reference:
Crystal structure of the dynamin tetramer
Thomas F. Reubold, Katja Faelber, Nuria Plattner, York Posor, Katharina Branz, Ute Curth, Jeanette Schlegel, Roopsee Anand, Dietmar J. Manstein, Frank Noe´, Volker Haucke, Oliver Daumke & Susanne Eschenburg; Nature (2015)