Capturing a New Conformation
A moment of laughter, candles being blown out on a birthday cake or the majestic flight of a bird overhead; capturing good, authentic images of living beings in motion is often a challenge. This task becomes increasingly more complex when your subject matter is gB, a fusion protein situated on the surface of the 200nm diameter Herpes simplex virus 1 (HSV-1), mediating the first step in the virus’ infection, i.e. entry into a cell. HSV-1 is a virus which is prevalent in approximately 90% of the adult population and manifests itself in about 30% of them as recurrent cold sore on an infected individuals lip or mouth. Using electron cryo-tomography, the team of CSSB group leader, Kay Grünewald and their collaborators from London were able to capture images of the gB protein in its native membrane context and discovered a hitherto elusive conformation of the glycoprotein which opens up opportunities for the development of novel interventions.
When a photographer in a portrait studio exclaims “say cheese” her subjects usually smile however, the resulting photos are often unnatural and the smiles appear a bit forced. Similarly, when scientists purify proteins for X-ray crystallization, they remove them from their natural environment and force them into a crystallized state. Purifying membrane proteins such as gB is particularly complex. In fact, ten years ago when scientists determined the structure of gB using X-ray crystallography several domains of the protein were removed in order to obtain a crystal. “The moment you try to purify this protein it essentially always goes into a stable post fusion, i.e. ‘job done’ form. This was the most difficult technical challenge for us to overcome,” explains Kay Grünewald “We need to trap the protein in a more native state.”
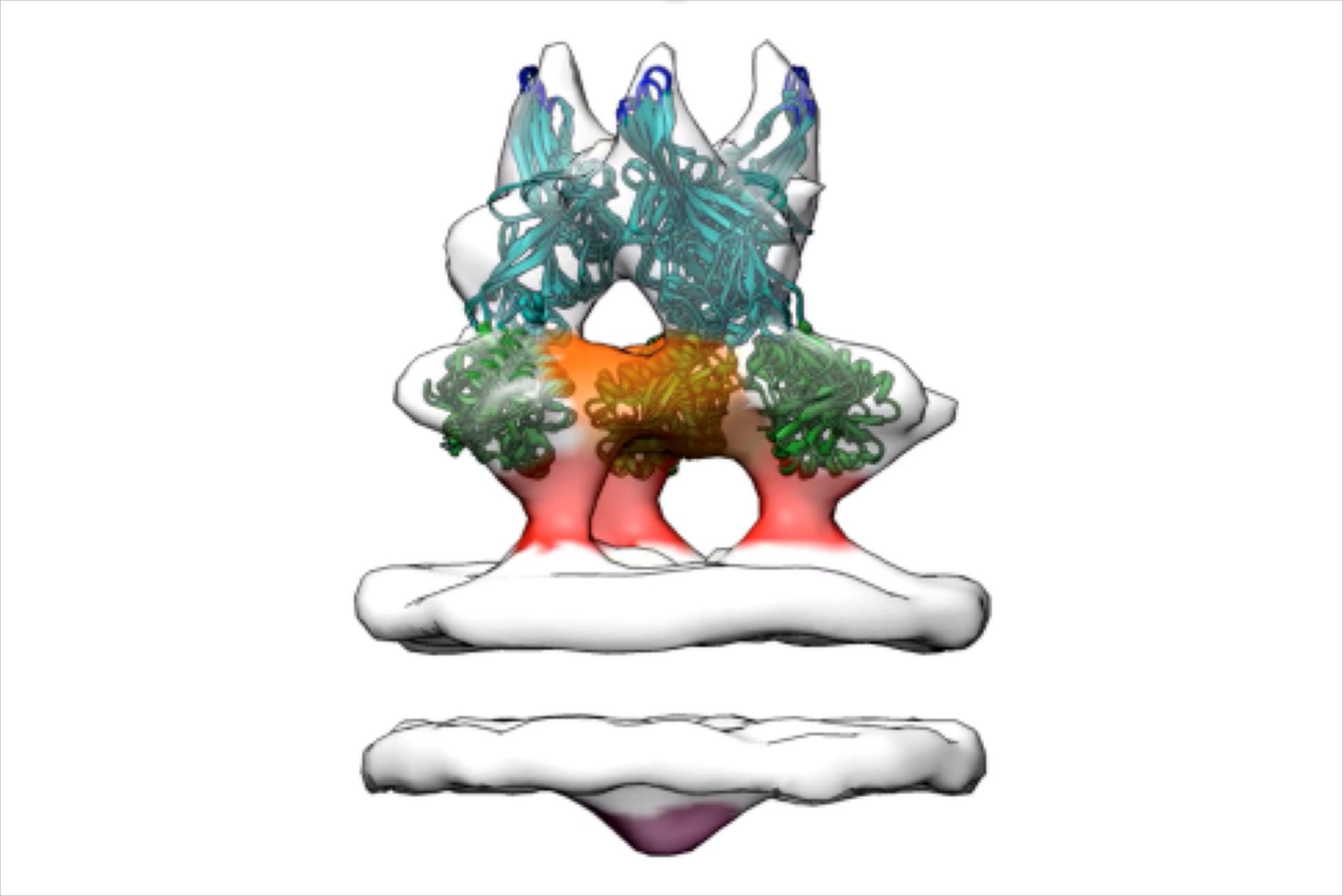
In an alternative approach, Grünewald and his collaborators decided to examine the glycoprotein in its full length and natively anchored in a lipid membrane using electron cryo-tomography. For this technique samples are rapidly immobilized in glass-like “vitreous” ice, a process which preserves their native structure. Using this approach allowed the scientists to image the protein in its full- length on cell-derived vesicles. “This was the trick that helped us break the deadlock that has existed in the field for quite some time and lead to the discovery of a new conformation,” clarifies Grünewald.
This new confirmation is provocative in that it exposes gB’s fusion loops to the outside thus providing a new insights into the herpesvirus fusion mechanism. “While it remains to be shown whether the new conformation is the sole pre-fusion conformation, it is for an understanding of the fusion process definitely highly relevant as we now have access to an ‘active’ conformation and not just the form which occurs after all the fusion action has been completed,” explains Grünewald. “We have presented our findings in conferences throughout the last year and people are extremely excited. I believe our finding will initiate new research and help many other labs in interpreting their hitherto unexplainable results.”
The scientific community is not the only group who is enthusiastic about this conformation; pharmaceutical companies are also extremely interested in this new discovery. Anti-viral creams are the most frequently used treatment for cold sores. These creams help the blisters heal faster but do nothing to prevent the virus from infecting the human host. “If you can interfere with this new conformation then you may be able to block the virus entry into the host cell in the first place,” clarifies Grüenwald “My lab is not involved in applied research however, there is keen interest from labs that will now try to use our results to design potential inhibitors or of raising new antibodies against this form.”
Grünewald’s lab will continue to focus on understanding the complex fusion machinery of HSV-1. “gB is just one player, we are trying to reconstitute and bring together the whole fusion machinery and see how the proteins interact. We would like to understand the full complexity of the process and I believe that we now have both the tools and knowledge to accomplish this.” The lab will also focus on pushing the structure of gB to higher resolution to reveal more of its details. “We are really proud of what we have accomplished so far,” states Grünewald “I am completely convinced that this is a milestone in the understanding of herpesvirus entry.”
Source Article:
Two distinct trimeric conformations of natively membrane-anchored full-length herpes simplex virus 1 glycoprotein B.
Zeev-Ben-Mordehai T, Vasishtan D, Hernández Durán A, Vollmer B, White P, Prasad Pandurangan A, Siebert CA, Topf M, Grünewald K. Proc Natl Acad Sci, 2016 Mar 24. pii: 201523234.



