Protein Assemblies Ejected from Native Membranes
An international team of scientists including CSSB’s Kay Grünewald (HPI, UHH) have developed an approach to eject protein assemblies directly from native membranes for mass spectrometry. This detergent-free, chemical free method not only enables the oligomerization state determination of individual membrane proteins but also reveals further functional information such as subunit composition in larger complexes and small-molecule binding. The group recently published their findings in a paper in Science.
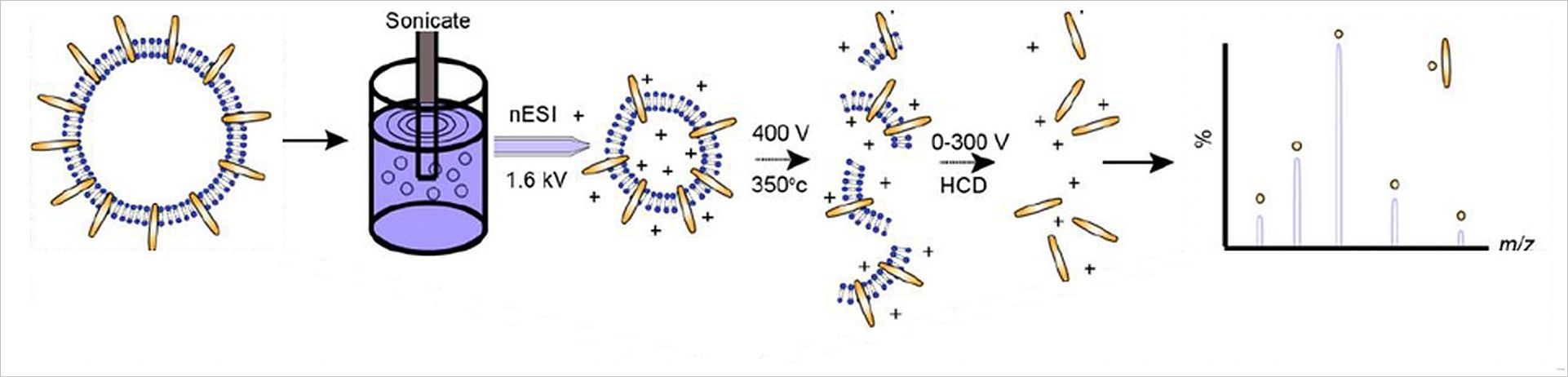
Membrane proteins exist in lipid bilayers and perform a number of functions essential to the survival of organisms. To conduct structural analysis, structural biologists typically extract the proteins from the lipid bilayer with the aid of detergents or chemicals. While these reagents enable the effective isolation of proteins, the integrity of the protein sample is often compromised and the functional context lost.
Grünewald and his collaborators discovered that exposing membrane vesicles to ultrasound vibrations through sonication destabilizes their integrity sufficiently to cause intact protein assemblies to be ejected from the lipid bilayer. After ejection, these protein assemblies are then sprayed into a mass spectrometer for measurement.
“What is truly unique about this method,” explains Grünewald “is that we are able to capture membrane proteins in their native state and that the proteins bring other molecules with them thus enabling us to catch a glimpse of the proteins functional interactions within the membrane.” This method points to new ways of studying not only the structure but also the function of membrane protein assemblies at an unparalleled mass resolution. The insights gained from this new approach could provide scientists with a more detailed understanding of the effects of pharmaceutical drugs on target complexes in their native membrane environments as well as elucidate the intricate functions carried out within the cellular membranes of prokaryotic and eukaryotic organisms.
While this new approach will be further refined and developed by Carol Robinson’s laboratory in Oxford, Grünewald sees the potential for the approach to advance research projects here at CSSB. “We plan to use this approach to help our investigation of virus fusion machineries,” explains Grünewald “but this approach will surely also be of interest to some of the other group leaders, as investigating membrane proteins is a major theme of research at CSSB.”
Original Publication:
Chorev DS, Baker LA, Wu D, Beilsten-Edmands V, Rouse SL, Zeev-Ben-Mordehai T, Jiko C, Samsudin F, Gerle C, Khalid S, Stewart AG, Matthews SJ, Grünewald K, Robinson CV. (2018) Protein assemblies ejected directly from native membranes yield complexes for mass spectrometry. Science. 16;362(6416):829-834



