Understanding Transporters: A Path to Improving Drug Transport Efficiency
Anything wishing to enter a cell must pass through its membrane which acts as selectively permeable barrier. Many small substrates such as dietary proteins, peptides and drugs are absorbed into the cell via membrane proteins called transporters. To improve the efficiency of drug uptake, CSSB scientist Christian Löw and his group would like to understand how membrane transport proteins works on the molecular level. Two recent publications in Cell Structure have brought his laboratory a step closer to accomplishing this.
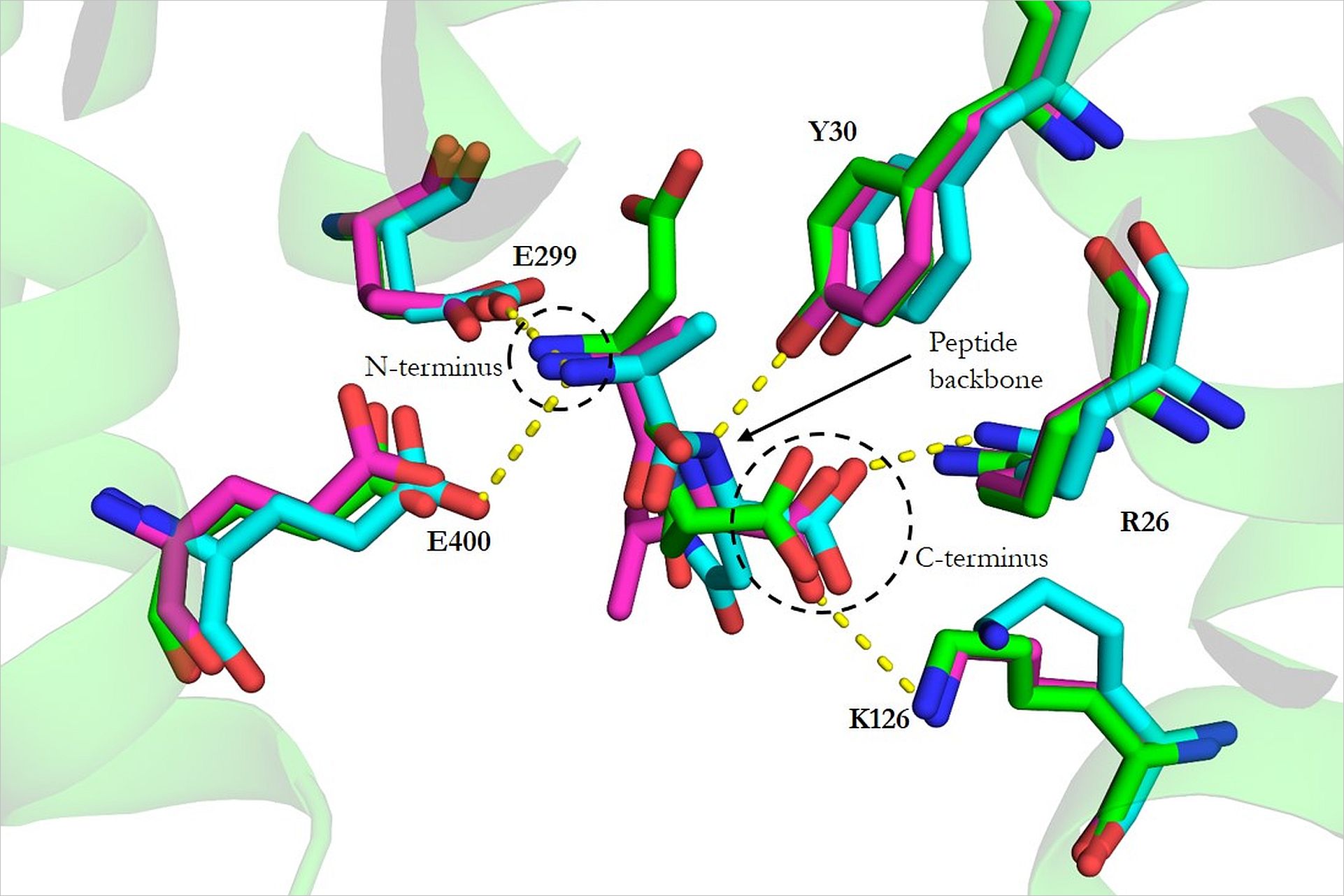
Adaptable substrate binding
The Löw group set out to understand the governing principles which allow Proton-Dependent Oligopeptide Transporters (POTs) to transport a wide-range of substrates. “POTs are promiscuous transporters,” explains Löw “They accept over 8,000 different substrates.” Using new instruments available at the CSSB’s Protein Characterization core facility (run in conjunction with EMBL Hamburg) Löw was able to screen a library of peptides for stability and binding affinities. Four dipeptides with substantial sequence variation were identified which could be effectively crystallized in complex with the transporter PepTST from the bacterium Streptococcus thermophilus.
Using crystallography data gathered at the P13 and P14 beamlines, Löw’s group was able to solve several high-resolution membrane structures thus shedding significant light on the structural basis for the broad substrate range of POTs. “We discovered that all substrates occupy the same binding site in the transporter,” explained Löw “This binding site is however extremely adaptable. Not only do the tranporter’s side chains adapt but the amount of water displaced within the structure also changes.” Moving forward, Löw hopes to be able to generate similar high-resolution structures for the protein in complex with other peptides and ultimately with drugs.
A new tool box
In addition to developing a better understanding of the molecular structures of transporters, the Löw group is actively developing new tools to study the structure and function of membrane proteins. “The biggest limitation of membrane protein research is that individual proteins often have to be taken out of the membrane with detergents which act as a poor mimic of a natural lipid membrane,” explains Löw “this is often an extremely difficult and time consuming task.”
In 2016, the Löw group helped demonstrate that saposin lipid nanoparticles (SapNPs) can be used to reconstitute membrane proteins in a detergent free environment. “The lipid binding protein saposin wraps around the transmembrane proteins in a doughnut like fashion,” explains Löw “This method not only creates detergent free membrane preparations but also stabilizes the protein. In addition, it allows the membrane proteins to maintain their native conformation.” The Löw group has now tested the SapNP tool systematically on different proteins in the presence of different lipids thus providing other scientist with a tool box for using this new method.
The SapNP technology allows scientists to work in a detergent free environment however; detergents were initially used to extract the proteins from the membrane. “Ideally we would like to eliminate the use of detergents entirely,” explains Löw. "These developments are ongoing and will allow us to study membrane proteins that cannot be extracted with detergents in the future. Furthermore, the SapNP method is beneficial for studying the structure of membrane proteins by single particle electron cryo-microscopy which avoids the tedious step of growing crystals.” The combination of both methods at the CSSB’s cryo-EM facility will provide new structural and functional information about membrane proteins such as the human PepT1 and PepT2 transporters, which transport drugs into human cells.
Original Publications:
Martinez Molledo M., Quistgaard E., Flayhan A., Pieprzyk J., Löw C., (2018) Multispecific Substrate Recognition in a Proton-Dependent Oligopeptide Transporter. Structure
doi:10.1016/j.str.2018.01.005
Flayhan A., Mertens HDT., Ural-Blimke Y., Martinez Molledo M., Svergun DI., Löw C. (2018) Saposin Lipid Nanoparticles: A Highly Versatile and Modular Tool for Membrane Protein Research. Structure
doi: 10.1016/j.str.2018.01.007



