Cryo-SOFI; a New Super-Resolution cryo-FM Concept
Correlative light and electron cryo-microscopy (cryo-CLEM) combines the powers of fluorescence cryo-microscopy (cryo-FM) with those of electron cryo-microscopy (cryo-EM) to answer important biological questions. The CSSB research groups of Rainer Kaufmann (UHH) and Kay Grünewald (HPI), together with coworkers from the University of Oxford, have developed a new cryo-FM concept which enables cryo-CLEM imaging that not only preserves the structural integrity of the biological sample but also significantly reduces the resolution gap. This new concept, named cryo-SOFI, has been published in the scientific journal PNAS.
While cryo-FM excels at pinpointing the exact location of individual labeled proteins, cryo-EM reveals high-resolution biological structures in their native environment. In cryo-CLEM experiments, both imaging techniques are applied to the same flash-frozen sample thus providing scientists with a more complete picture of the macromolecular landscape in the cell. “Cryo-CLEM aims to take full advantage of the complementary features of both imaging modalities,” explains Kaufmann “there are; however, several technical challenges that need to be overcome.”
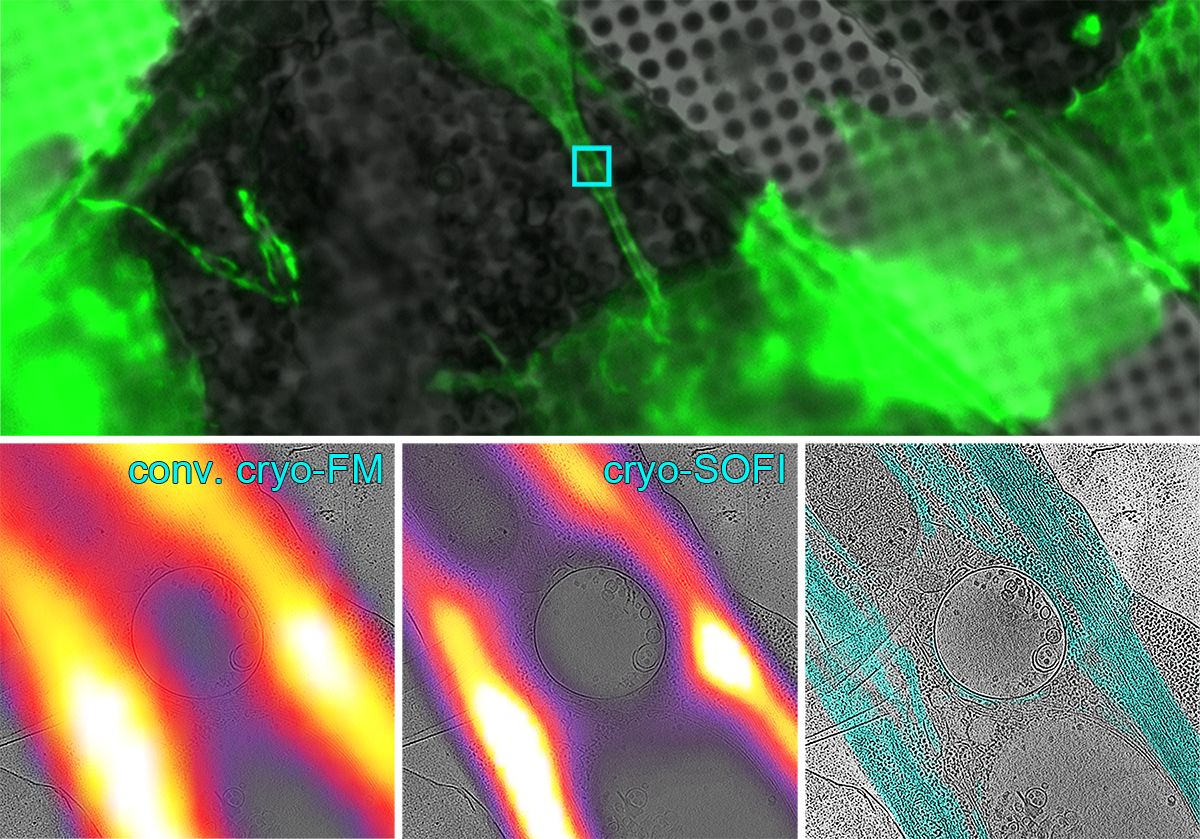
The new cryo-SOFI concept provides a general solution to the technical challenge of specimen devitrification which occurs when a sample warms to above -135 degrees Celsius. The high laser intensities typically needed for super-resolution cryo-FM imaging warm up the specimen and can cause the specimen’s amorphous ice to transition into a crystalline form. This damages the specimen’s structure and results in a loss of contrast in the subsequent cryo-EM images.
Based on the SOFI principle (super resolution optical fluctuation imaging), cryo-SOFI uses low laser intensities that are able to keep specimens below the devitrification temperature threshold. Using a reconstruction algorithm that makes use of fluorescence intensity fluctuations in the sample over time, cryo-SOFI is able to attain resolutions of approximately 135 nm. This is a threefold improvement over conventional cryo-FM methods and the biological structure of the specimen is preserved for cryo-EM imaging.
“The most exciting aspect of the cryo-SOFI concept is its simplicity,” clarifies Kaufmann “there is no special sample preparation needed and no complex optical set-up is required.” The cryo-SOFI concept is compatible with all broadly used fluorescent molecules and suitable for all types of specimens. Kaufmann and Grünewald see wide applications for this concept and find that it will be particularly useful in imaging structures residing in thicker parts of cells as it is able to eliminate out of focus signals.
Original publication:
Moser, Pražák et al. (2019) Cryo-SOFI enabling low-dose super-resolution correlative light and electron cryo-microscopy. PNAS