First Structure of Peptide Transporter in Complex with Pro-drug Revealed
Certain drugs and pro-drugs can hijack the human nutrient transporters PepT1 or PepT2 and hitch a ride directly into the cell thus accelerating the drug’s absorption into the blood stream. Pro-drugs are inactive medications that are metabolized into an active, functional form within the body. While the pro-drug concept is very effective, little is known at the molecular level about the peptide transporters’ structure and how they recognize, bind and transport pro-drugs. The groups of CSSB scientists Christian Löw (EMBL) and Jan Kosinski (EMBL) are now a step closer to understanding this as they recently determined a high resolution crystal structure of a peptide transporter in complex with the pharmacological relevant pro-drug valganciclovir; a medication that combats certain viral infections. Their results, published in the Journal of the American Chemical Society, could assist in the design of pro-drugs with improved absorption rates.
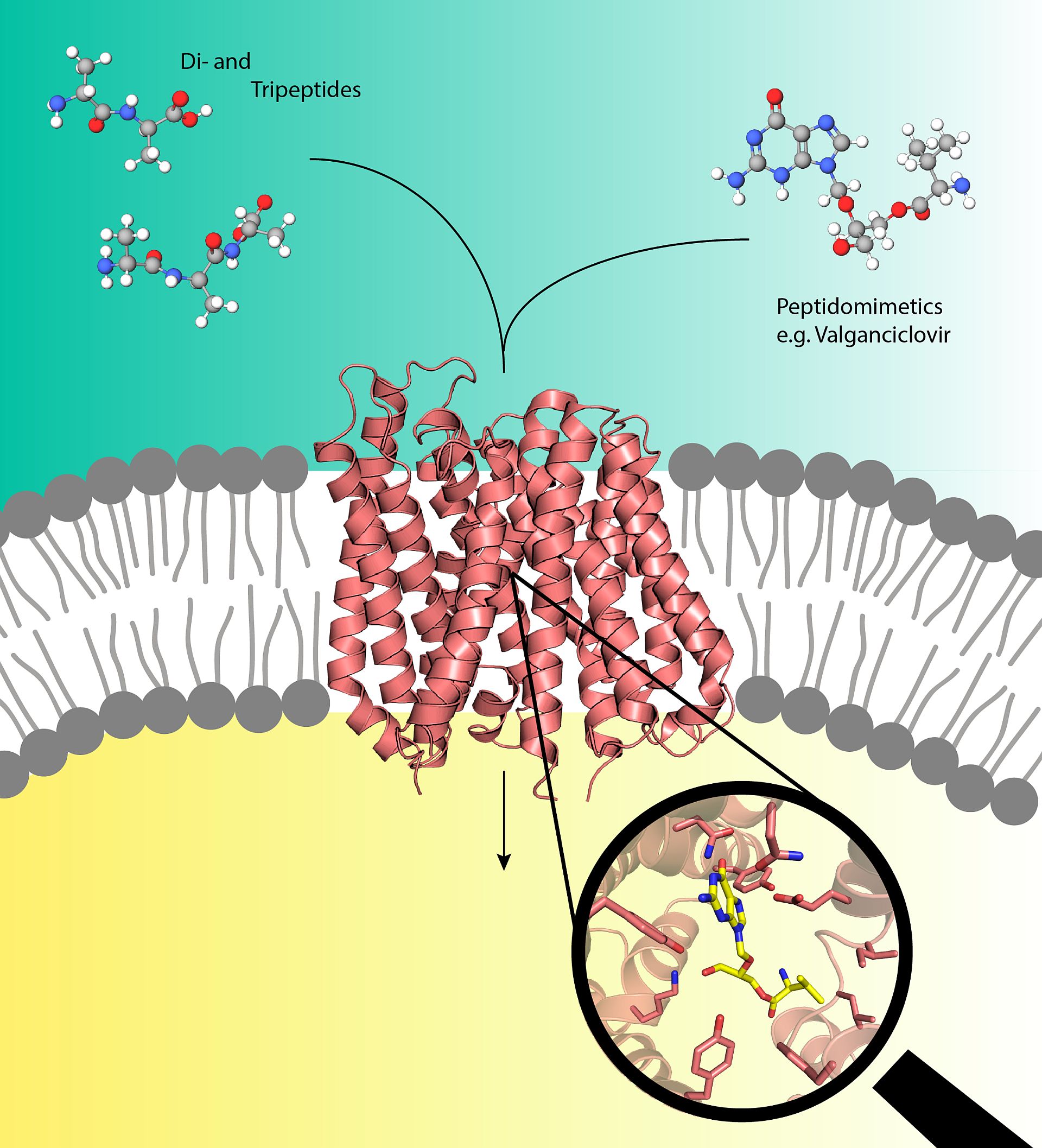
The bacterial peptide transporter DtpA from E. coli is a close homologue of the human PepT1 transporter and was therefore chosen by the scientists as a prototype. To produce well-diffracting crystals of DtpA, Loew teamed up with the group of Jan Steyaert from Belgium to generate DtpA specific nanobodies. Nanobodies are single-domain antibody fragments, which are derived from the naturally-occurring heavy chain only antibodies found in llamas. “The nanobodies act as crystallization chaperones”,” explains Löw. “They increase the stability of membranes proteins such as DtpA thus allowing us to generate high quality crystals.”
The Löw group then carried out the crystallization experiments of DtpA in complex with a nanobody and a pro-drug molecule at the Sample Preparation and Characterization facility (SPC) and analyzed the obtained crystals at the EMBL operated beamlines P13 and P14 which are part of DESY's X-ray light source PETRA III. The scientists were able to determine a 2.65 Ångstrom resolution structure of DtpA bound with the pro-drug valganciclovir that revealed an unexpected binding mode. “The orientation of the prodrug in the binding pocket is flipped by 180 degrees relative to prior predictions,” explains Löw “We also discovered that DtpA preferably binds and likely transports tripeptides over dipeptides.”
The Kosinski group built a high-quality structural model of the human PepT1 transporter in complex with valganciclovir using the DtpA crystal structure as a guide. “The model shows that human PepT1 very likely binds the drug in the same way as DtpA and pinpoints the exact amino acid groups responsible for binding,” clarifies Kosinski. Therefore, the insights generated by this model of PepT1 may facilitate development of new pro-drugs with improved absorption rates. Such improvements would not only lower the pharmacologically effective dose but could also reduce the negative side effects experienced by patients.
Reference
Structure of Prototypic Peptide Transporter DtpA from E. coli in Complex with Valganciclovir Provides Insights into Drug Binding of Human PepT1; Yonca Ural-Blimke, Ali Flayhan, Jan Strauss, Vasileios Rantos, Kim Bartels, Rolf Nielsen, Els Pardon, Jan Steyaert, Jan Kosinski, Esben M Quistgaard, and Christian Löw; Journal of the American Chemical Society, 2019



