Antiviral drug, Favipiravir, could kill SARS-CoV-2 viral genome
CSSB Scientific Director, Chris Meier(UHH), and his postdoc Johanna Huchting were involved in a collaborative study with researchers from AFMB-CNRS, the University of Aix-Marseille, Universität Hamburg, and Colorado State University that reported the first detailed kinetic and mechanistic analysis of SARS-CoV-2’s polymerase enzyme. Using the antiviral drug Favipiravir (Avigan), the researchers have managed to make the virus accumulate too many mutations, which overloads its error correcting machinery and ultimately renders the viral genome non-functional.
The SARS-CoV2 virus is part of the Coronavirus family which has seven members that infect humans and of these only SARS-CoV-1 and 2 and MERS-CoV are highly pathogenic. SARS-CoV-2 is the most contagious coronavirus as demonstrated by the current pandemic which has resulted in more than 30 million infections and almost one million deaths.
The coronaviruses use RNA as their genetic material and have genomes that are 3-4 times larger than the "classic" RNA viruses (Dengue, Flu, Ebola, Hepatitis C, Polio). The coronavirus genome encodes for many viral proteins, some of which allow the viruses to evade the cell's immune response, while others allows the virus to grow by replicating their genomes. A central protein of the replication process is the viral RNA polymerase, an enzyme that has no functional equivalent in human cells and is therefore a very interesting target for antiviral treatments.
For some viruses, including HIV, hepatitis C, herpes viruses and influenza virus, drugs exist that target the viral polymerase in the form of nucleoside analogues. These compounds can be incorporated into the viral RNA by the polymerase in place of its four natural genetic bases, thus stoping further synthesis of the genome and ultimately killing the virus. Coronaviruses are, however, unique in that they have an added exonuclease enzyme that is capable of repairing the damage caused by many of these drugs, reducing their antiviral utility. The antiviral drug Remdesivir is at least partially resistant to this repair, contributing to its success against SARS-CoV-2, but it must be administered intravenously and its dosage is delicate.
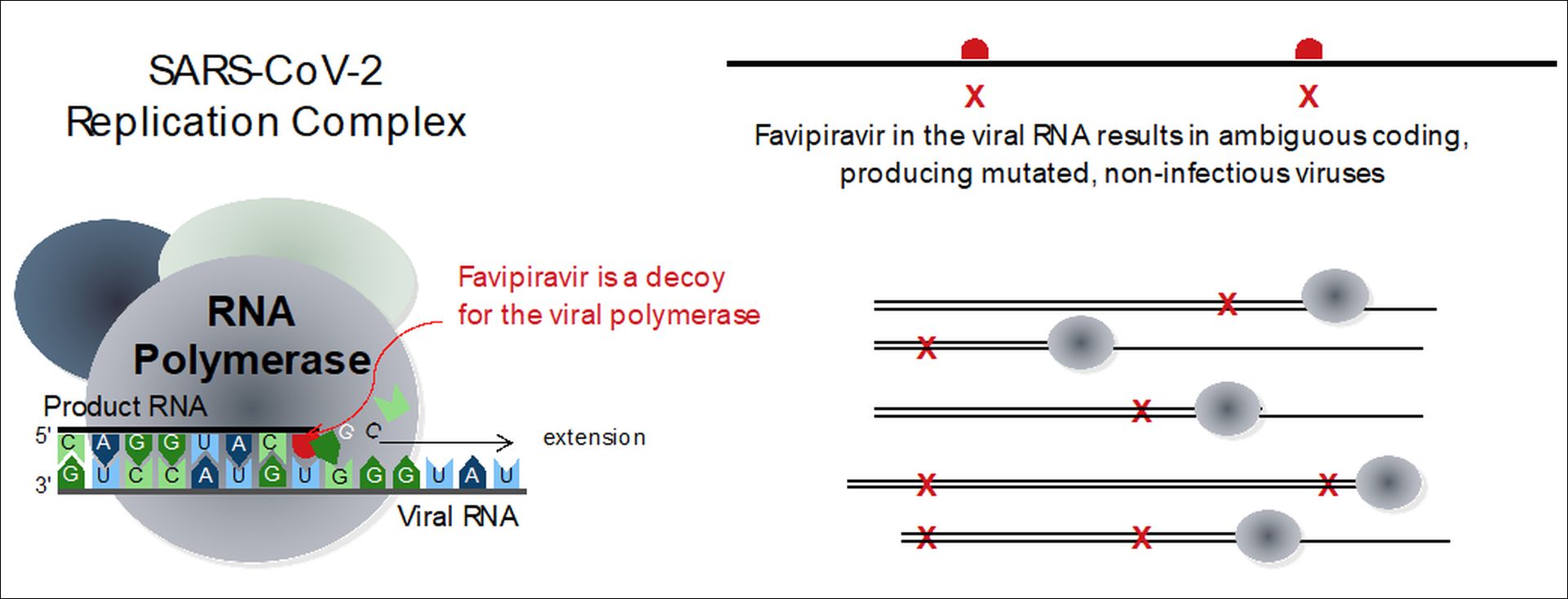
Researchers from AFMB-CNRS, the University of Aix-Marseille, Universität Hamburg, and Colorado State University report the first detailed kinetic and mechanistic analysis of the coronavirus polymerase enzyme using the anti-influenza drug Avigan (i.e. Favipiravir or T-705). Avigan is already on the market in Japan and in clinical trials for several months in Russia, China, Iran, India, Japan and the United States. It does not stop the synthesis of viral RNA, but rather allows the synthesis of genetically altered genomes, which pushes the virus towards an evolutionary and functional impasse and ultimately produces an antiviral effect via a mechanism called “error catastrophe.”
The researchers discovered two quite remarkable properties of the SARS-CoV-2 RNA polymerase: in order to synthesize SARS-CoV-2’s very long genomes, the RNA polymerase has evolved an extraordinarily high catalytic efficiency and replicates the RNA at very high speeds that are about ten times faster than similar RNA virus polymerases. At the same time, and under normal conditions of infection, the data show the polymerase has sacrificed its capacity for genetically faithful synthesis, which is possible because the coronaviruses have added an error-repairing exonuclease enzyme that can help restore a faithful copy of the genome.
From these results, a new concept emerges that could impact the design of anti-coronavirus drugs. Using nucleoside analogue compounds that look similar to the normal genetic bases these drugs could take advantage of the extraordinary low fidelity of the coronavirus polymerase and deceive the enzyme into synthesizing a lot of errors. If so many errors are produced that the error correcting mechanism is overloaded, the virus would then "over-mutate" until it dies. This approach may be more efficient than trying to stop both the viral synthesis and repair steps of SARS-CoV-2 simultaneously.
Reference:
Shannon A, Selisko B, Le NT, Huchting J, Touret F, Piorkowski G, Fattorini V, Ferron F, Decroly E, Meier C, Coutard B, Peersen O, Canard B. (2020) Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat Commun. 11(1):4682. doi: 10.1038/s41467-020-18463-z