Herpes viruses: New insights into the fusion process upon entry into the host cell
In a collaborative study conducted by the Heinrich Pette Institute, Leibniz Institute for Experimental Virology (HPI), the Centre for Structural Systems Biology (CSSB), the University of Oxford, the Birkbeck University of London, the Friedrich Loeffler Institute and the Institut Pasteur, the fusion process of herpes viruses with the host cell during cell entry was analyzed in detail using a multi-methodological approach. The main focus of the investigation was the prefusion structure determination of the herpes simplex virus 1 (HSV-1) glycoprotein B. The results have now been published in the renowned journal Science Advances.
On the membrane surface of all enveloped viruses, specialized proteins catalyze the fusion between the viral end cellular membranes to enable virus entry into the host cell. In herpes viruses like herpes simplex virus 1, this membrane fusion is achieved by structural rearrangement of these proteins from a metastable prefusion conformation to a stable postfusion conformation.
The prefusion form is an attractive target for the drug and vaccine development as it is the prevalent conformation on the infectious virus. However, the metastability makes this protein highly unstable, causing it to easily transform into the defused postfusion form. Thus, identification of ways to stabilize the prefusion conformation is an important step for structure determination and hence for drug and vaccine development.
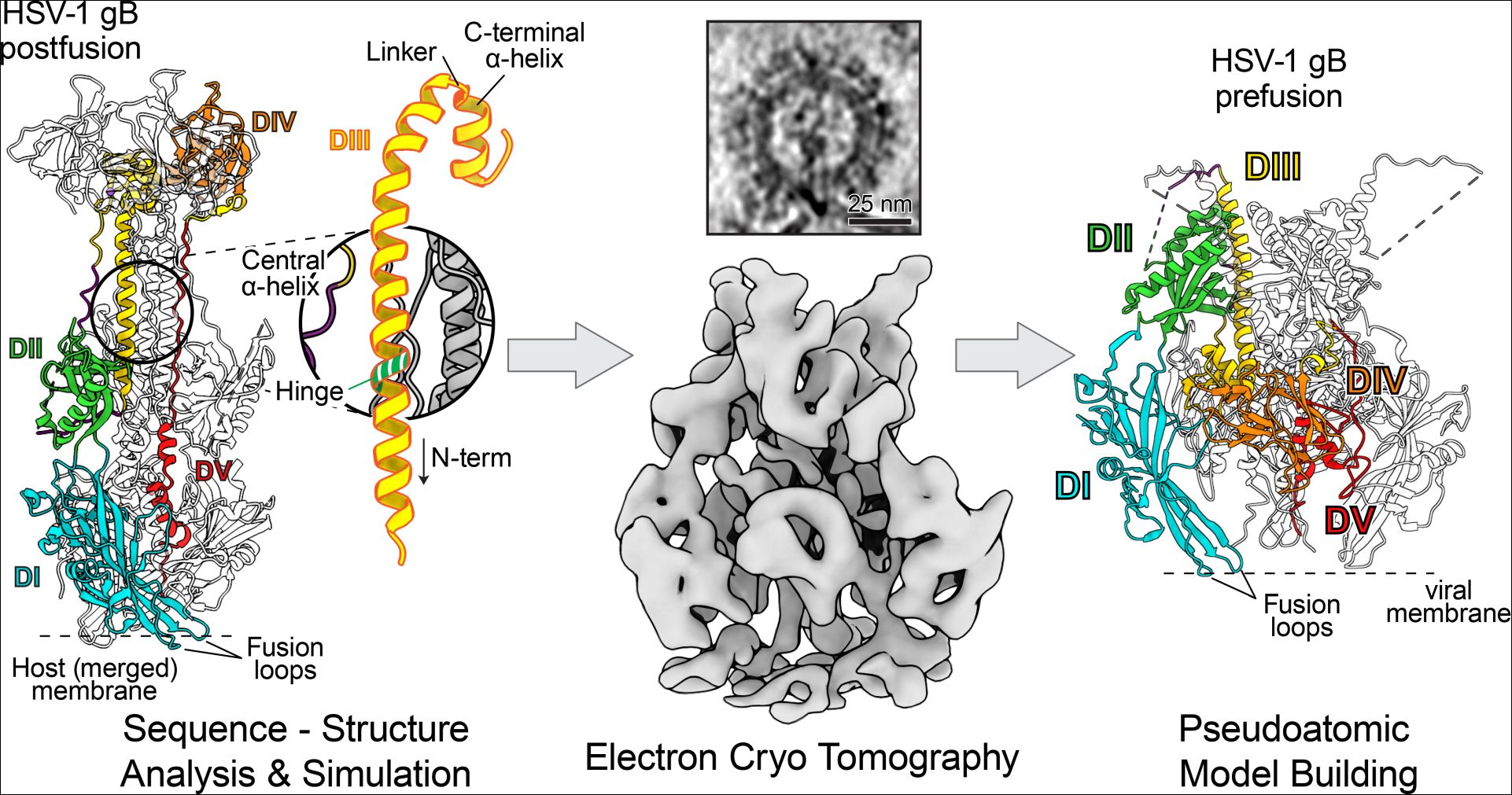
The now presented publication focuses on the membrane fusion glycoprotein B (gB) of the herpes simplex virus 1. Together with the group of Prof. Maya Topf (Birkbeck university) conserved sequence signatures of this class of fusion proteins were identified. This crucial information allowed molecular dynamics simulations, which successfully predicted the position for a functional mutation which locks the protein in its prefusion form. Functional and structural tests were then carried out with the group of Prof. Thomas Mettenleiter (Friedrich Loeffler Institute) and the group of Prof. Felix Rey (Institut Pasteur) to characterize this interesting mutant further. The final determination of this elusive structure with a resolution in the sub-nanometer range was only possible using electron cryo tomography.
The results allow a detailed description of gB’s domain arrangements, which reveal the substantial structural changes required to drive the membrane fusion reaction. In addition, there is a remarkable similarity to the conformational changes seen for the glycoprotein G of the vesicular stomatitis virus. "Although both proteins originate evolutionarily from seemingly unrelated virus families, the arrangement and structures of their individual domains are conserved", explains Prof. Kay Grünewald, who headed the study.
Both, fusion glycoprotein B and G, are class III fusion proteins: "Our comparative sequence-structure analysis, therefore suggests a common domain rearrangement in all class III viral fusion proteins. Antibodies or antiviral drugs blocking this conformational change could provide clinically relevant protection. Our results thus represent an important step towards the development of new drugs against human pathogenic herpes viruses", summarizes first author Dr. Benjamin Vollmer.
Reference:
Vollmer B, Pražák V, Vasishtan D, Jefferys EE , Hernandez-Duran A, Vallbracht M, Klupp B, Mettenleiter TC, Backovic M, Rey FA, Topf M, Grünewald K (2020). The prefusion structure of herpes simplex virus glycoprotein B. Science Advances. doi: 10.1126/sciadv.abc1726