Needle Complex Structure Revealed
According to the World Health Organization diarrhoeal diseases caused approximately 1.4 million deaths in 2016. The bacterial pathogen Shigella was responsible for 13% of these deaths making it the second leading cause of diarrhoea mortality worldwide. For successful infection of human host cells, Shigella and other Gram negative bacteria use a large macromolecular complex called the type III secretion system (T3SS).
The T3SS’s syringe-like needle complex is responsible for the delivery of effector proteins into the cytosol of target cells. Since the effectors help the bacteria to invade host cells and to circumvent cellular defense mechanisms their controlled release through the needle complex is essential for the bacteria’s survival. The CSSB group of Michael Kolbe focuses on understanding the molecular mechanisms of the T3SS. In a recent publication in PLoS Pathogens, the Kolbe group presented the first cryo-EM Structure of the isolated Shigella needle complex which reveals new insights into the architecture and function of this essential virulence factor.
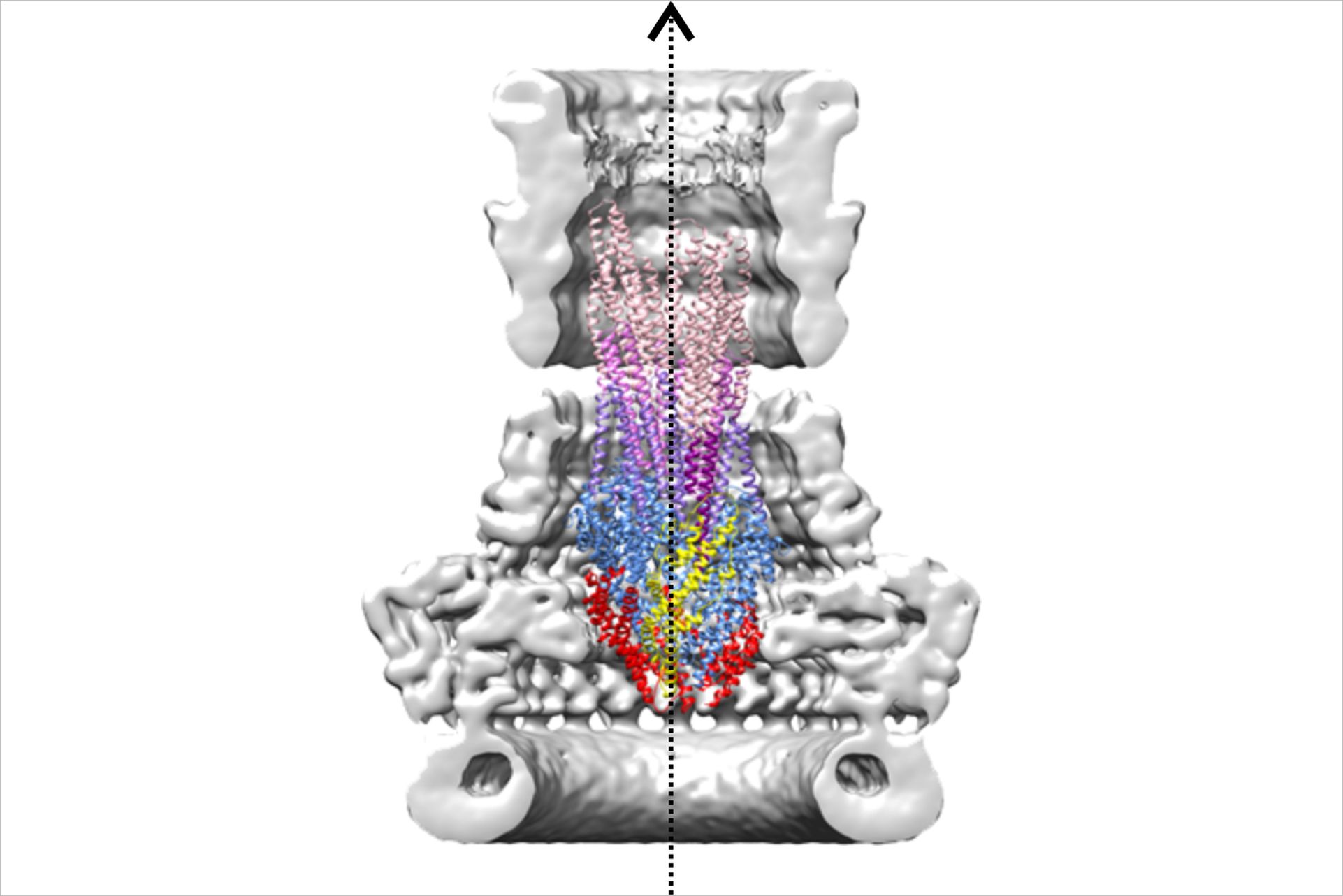
High-resolution cryo-EM maps enabled the elucidation of the Shigella needle complex’s atomic structure. The structure of this large complex explains the assembly and the interaction of its protein subunits. “The map of the needle complex revealed a mixed C15/C16 symmetry of the outer membrane spanning secretin oligomer,” explains Antje Kamprad, one of the paper’s lead authors “This asymmetry illuminates how the secretin can connect to the inner membrane ring to build a functional needle complex. Another important result of this study is the identification of a network of water filled channels crossing the inner membrane ring in Shigella and Salmonella needle complexes suggesting an important function for the secretion of effector molecules”.
Overall, the high resolution maps provide essential details that help contribute to a better understanding of this important virulence factor. “These structural insights into the mechanisms of T3SS will ultimately help in the development of future strategies and novel therapeutics against Shigella.” explains Michael Kolbe.



