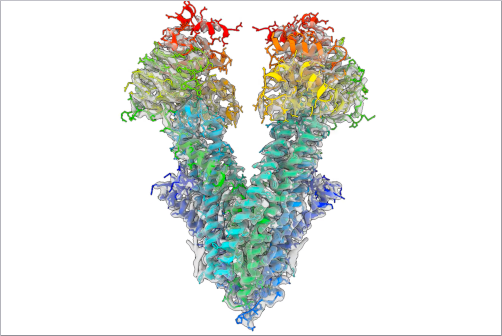
Deciphering two antimicrobial amyloid structures using cryo-electron microscopy.
Researchers from CSSB and collaborators have deciphered the structure of two antimicrobial peptides (AMPs) from frogs. The AMPs act as a toxin against microbes and have a similar structure to human protein fragments associated with Alzheimer's and Parkinson's disease, respectively. The findings were published in July 2022 in the journal Nature Communications.
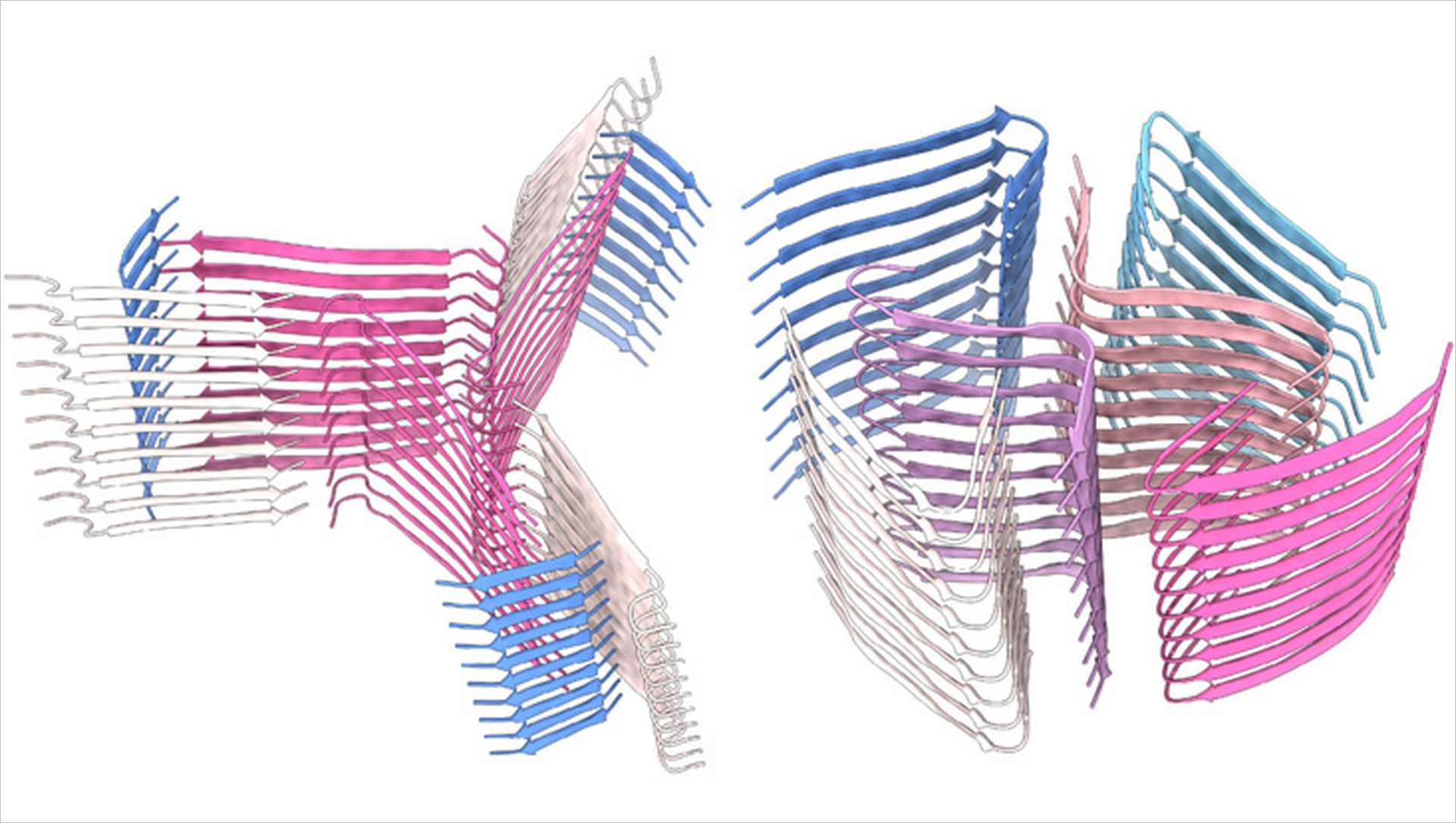
Antimicrobial peptides (AMPs) are molecules that fight microorganisms such as bacteria and have been present in most organisms since the dawn of life. Scientists have already identified thousands of different AMPs. Certain AMPs assemble into well-ordered fibrils, which are bundles of long, threadlike molecules. In terms of their arrangement, these AMPs resemble amyloids, small protein fragments that are produced by the body and can be deposited in human tissue. They are therefore also associated with neurodegenerative and systemic diseases such as Alzheimer's and Parkinson's. Some human amyloids are, however, functional and serve, for example, to store hormones or encapsulate RNA. In addition, various other organisms, e.g. bacteria and toads, also secrete amyloids, which can then act as cell toxins.
Scientists from the Universität Hamburg (UHH), the European Molecular Biology Laboratory (EMBL) Hamburg and the Centre for Structural Systems Biology (CSSB), together with researchers from the Technion - Israeli Institute of Technology in Haifa, have used cryo-electron microscopy (cryo-EM) to investigate the structures of two AMPs from amphibians, namely uperin 3.5 and aurein 3.3, in more detail. Uperin 3.5 is found on the skin of certain toad species and, simply put, decorates them with 'spirelli' shaped fibers. Through interaction with microbes, the 'spirelli' shaped fibers can change to a different structure, that is 'macaroni' shaped. This structural change could potentially cause the fibers to interact differently with microbes and destroy the microbial membrane, making it toxic.
"Surprisingly, the cryo-EM structures showed that both urein 3.5 and aurein 3.3, have cross-β-amyloid structures," said Dr. Carolin Seuring, head of the cryo-EM facility at CSSB and a research associate at the Department of Chemistry at the Universität Hamburg. "Aurein 3.3 has kinks that are the hallmark of so-called low-complexity amyloid-like reversible kinked segments (LARKS), which are found in functional human amyloids." Because of the similar structure of human amyloids and amphibian AMPs, the researchers therefore hypothesize that human amyloids, which are known to be purely pathological, may also have antimicrobial activity.
"Of course, further extensive studies are needed to investigate the structure-function relationship and mechanisms in more detail," said Prof. Meytal Landau of the Technion, a principal investigator involved the study and an associate member of CSSB as well as a visiting scientist at EMBL Hamburg. "However, our results support the link between amyloids and antimicrobial activity at the structural level. In addition, AMPs that form stable fibrils as amyloids may open up avenues for developing antimicrobial drugs with improved bioavailability, adhesion to surfaces and longer shelf life."
Original Publication
Bücker, R., Seuring, C., Cazey, C. et al. The Cryo-EM structures of two amphibian antimicrobial cross-β amyloid fibrils. Nat Commun 13, 4356 (2022). https://doi.org/10.1038/s41467-022-32039-z
Original News Story:
https://www.min.uni-hamburg.de/ueber-die-fakultaet/aktuelles/2022/0822-zwei-antimikrobielle-amyloidstrukturen.html