How do molecular machines pass the ball?
CSSB researcher Dr. Badri N. Dubey (DESY) from the Sondermann group, together with collaborators from Biozentrum, University of Basel, Switzerland, has revealed the molecular mechanism by which proteins pass a signal via a multi-step relay in a signaling pathway. The results have been published in the journal Nature Communications.
Human beings are constantly bombarded with information from their environment. Our nervous system processes these external stimuli and guides our body’s responses. For example, if we touch a flame, signals are instantaneously sent through the nervous system to our brain, which registers the heat and sends feedback causing us to move our hand. Bacteria process external stimuli in a similar way despite the lack of elaborate nervous system.
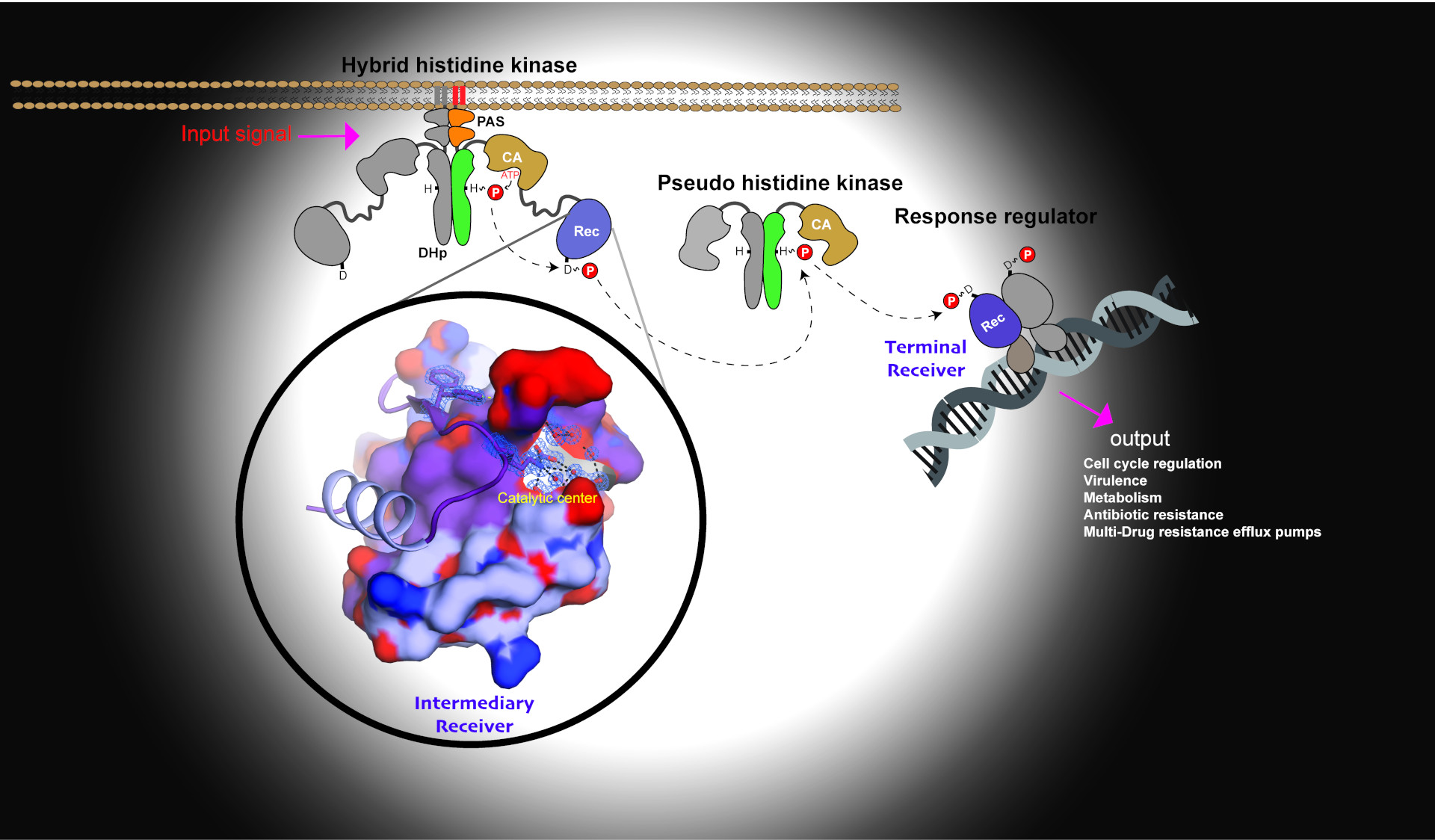
Signal transduction via phosphate molecule (red ball) in the multi-component signaling pathway. The crystal structure of intermediary Rec exhibits activated conformation shown in the inset.
Bacteria achieve this through complex signaling systems such as two-component system (TCS) pathways. The primary receptors of external signals are proteins called histidine kinases. The information gathered by the histidine kinases (HKs) is relayed to cognate response regulators (Rec term) ultimately resulting in the implementation of cellular programs such as gene transcription.
In this study, the researchers examined a poorly understood group of Rec domains (Recinter), that act as intermediaries in multistep phosphorelays. Using X-ray crystallography and NMR spectroscopy researchers determine that Recinter is not dimerized upon phosphorylation and that no structural changes occur. “In fact, the crystal structure revealed that Recinter’s active site remains in the active conformation and indeed no allosteric response was observed upon post-translational modification” explains Mitchell Brüderlin, first author of the paper.
“To use a football analogy, intermediary Recs act like a midfielder making an excellent pass without elaborate footwork or movement,” explains Dubey. “Recinter is essentially passing the ball (signaling information in the form of phosphate) onto the next player and finally to Recterm so that it can score a goal (dimerize and initiate gene transcription). It’s an integral part of the team but not the superstar.
The insights gained from this study elucidate the structural consequences of signal transmission for different players and is a key step in understanding a highly complicated signaling system. In future studies, the researchers plan to examine the nature of the interactions between these players at a molecular level. “Using all of this information, we could potentially block the transmission of signals, which would provide a route to novel antibacterial agents for the fight against biofilm formation in antibiotic-resistant infections,” notes Dubey.
Source:
Brüderlin, M., Böhm, R., Fadel, F. et al. Structural features discriminating hybrid histidine kinase Rec domains from response regulator homologs. Nat Commun 14, 1002 (2023). https://doi.org/10.1038/s41467-023-36597-8



