The power of two rings
Unveiling the secrets of bacterial second messenger dimerization
Second messenger signaling is a response to external stimuli that takes place in all domains of life. In gram-negative bacteria, the second messenger c-di-GMP is a central regulator of many crucial bacterial processes including the formation of biofilms and virulence. CSSB researchers Dr. Badri N. Dubey (DESY) and Prof. Holger Sondermann (DESY) in collaboration with scientists from Biozentrum, University of Basel, Switzerland, have revealed a mechanism of how a signaling protein imparts step-wise c-di-GMP dimerization as part of the signal transduction process. The results have been published in the journal Scientific Reports.
When two c-di-GMP molecules come together they form a dimer resembling two interlocked rings. Such a formation is prevalent when the second messenger is bound to certain signaling proteins, but less populated in solution. Whether these signaling proteins facilitate c-di-GMP dimerization as part of their regulatory mechanism or merely select rare, preformed c-di-GMP dimers from the solution is an open question.
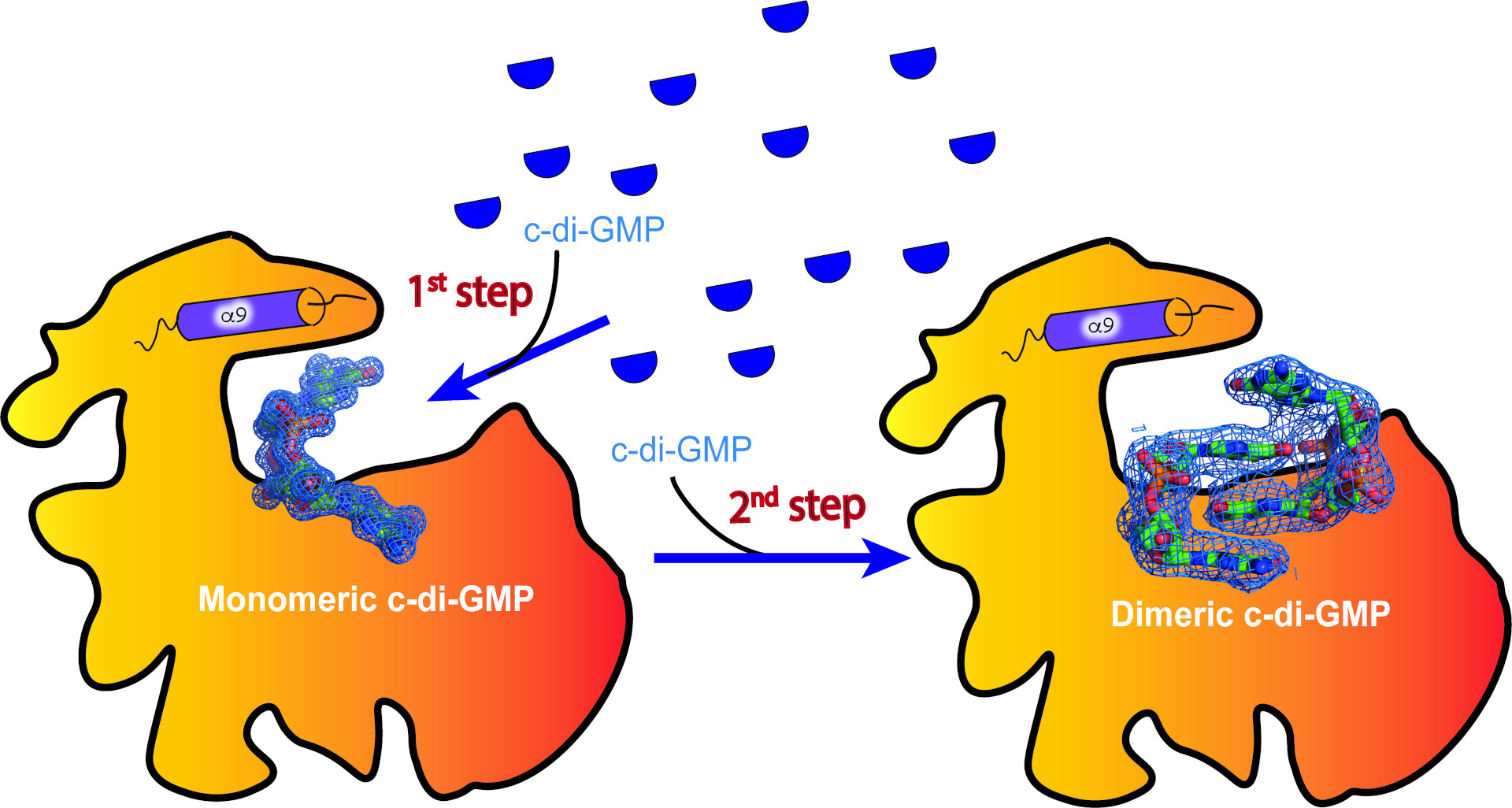
One example of such a signaling switch utilizing a c-di-GMP dimer is a metabolic switch protein SmbA from the Gram-negative bacteria Caulobacter, for which a previous study provided high-resolution structural information for the c-di-GMP-bound state. This new study now sheds light on a potential intermediate step, where a single c-di-GMP binds to SmbA first and then the c-di-GMP dimer assembles in a second step.
To understand this dimerization process, the researchers first designed a mutant form of the c-di-GMP-binding protein by deleting the dimer-inducing motif on a signaling protein. "When we determined the crystal structure of this mutant protein in the presence of c-di-GMP, we realized that we arrested the binding process at an intermediate step that had not been observed before” explains Dubey, corresponding author and project leader of the study. “Only one molecule of c-di-GMP was bound, ready to accept the second dimer half.”
"These new results, based on the mutant structure and accompanying biochemical validation, provide strong support for a two-step process of consecutive c-di-GMP binding at the singaling protein’s binding site. We propose that the protein’s surface serves as a template to prime dimer formation," explains Dubey. Considering that many signaling proteins prefer c-di-GMP in its dimerized form, it is conceivable that protein-primed, step-wise c-di-GMP binding represents a more widely used mechanism.
"Further experiments are needed to investigate the generalizability of the findings reported here,” notes Sondermann “This study describes key insights into step-wise c-di-GMP binding to establish a signaling switch in a particular protein, and thus provides the framework for future studies of the process.”
Source:
Dubey BN, Shyp V, Fucile G, Sondermann H, Jenal U, Schirmer T (2023) Mutant structure of metabolic switch protein in complex with monomeric c-di-GMP reveals a potential mechanism of protein-mediated ligand dimerization. Sci Rep 13, 2727 https://doi.org/10.1038/s41598-023-29110-0



