Breakthrough in understanding the Herpes simplex virus 1
The groups of CSSB Associate Member Martin Hällberg and CSSB group leader Kay Grünewald have made a breakthrough in understanding the molecular mechanics of the Herpes simplex virus 1 (HSV-1) DNA polymerase, shedding light on the enzyme's complex structural changes during DNA synthesis and proofreading. This comprehensive study employed advanced electron cryo-microscopy (cryo-EM) to capture high-resolution images of the HSV-1 UL30 DNA polymerase in three distinct functional states. These states are pivotal for the enzyme's activity and were examined to elucidate their roles in the viral replication process.
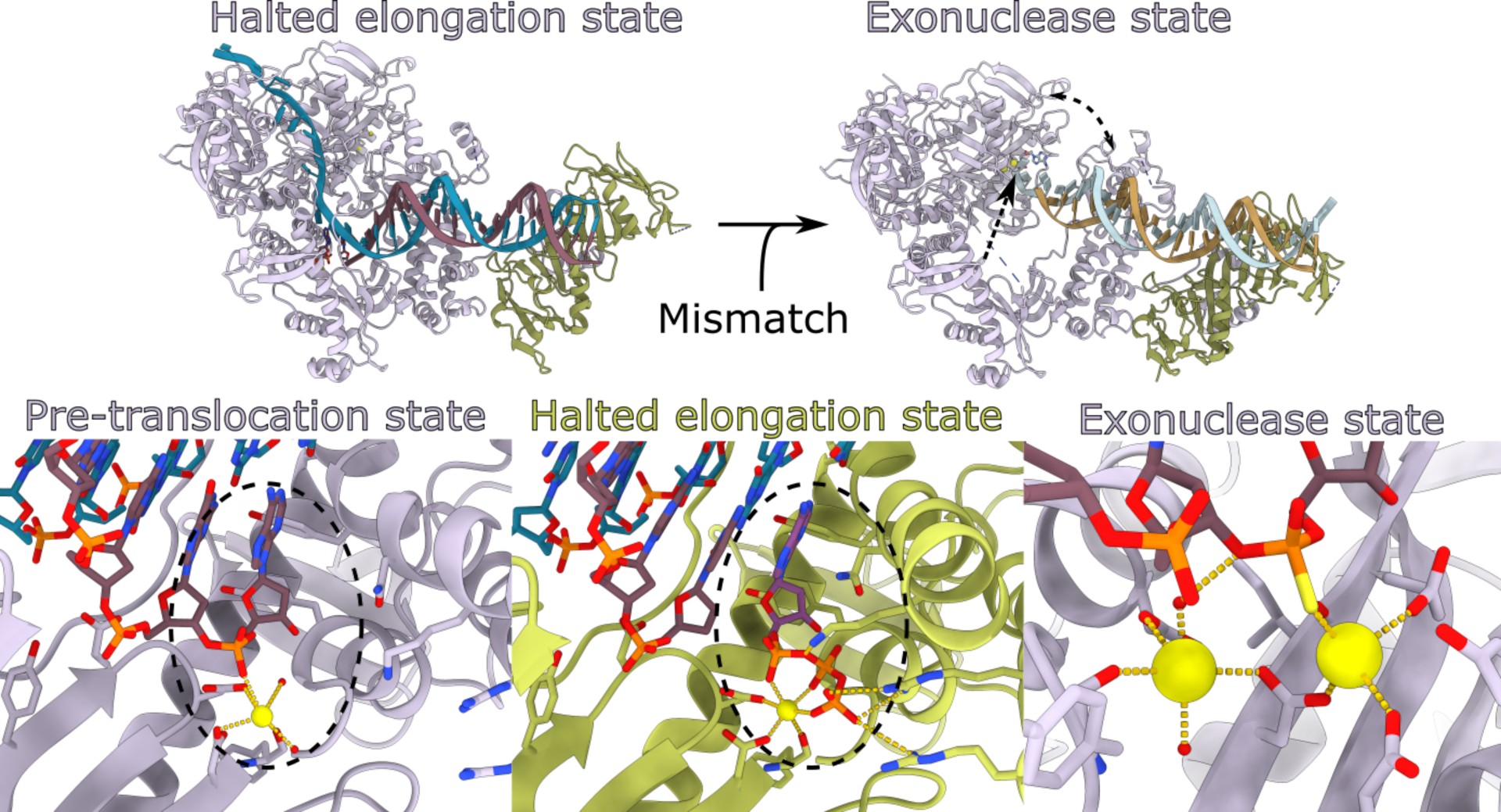
The implications of these findings are far-reaching, particularly in the context of antiviral drug development. HSV-1 is a widespread pathogen that can cause severe health issues, especially in immunocompromised individuals where resistance to existing treatments like acyclovir is a growing problem. By revealing the detailed structural dynamics of the HSV-1 DNA polymerase, this research opens up new avenues for designing targeted antiviral therapies that can inhibit the enzyme's function more effectively and potentially overcome existing drug resistance.
This study exemplifies the power of cryo-EM in capturing the intricate details of molecular machines in action. The high-resolution structures obtained provide a vivid depiction of the enzyme's functional states, contributing to a deeper understanding of the fundamental mechanisms underlying viral DNA replication. These insights are crucial for developing new strategies to combat HSV-1 infections and improving therapeutic outcomes for patients affected by this pervasive virus.
For more detailed information, the study is published in Nucleic Acids Research, accessible via DOI: 10.1093/nar/gkae374. The work was financed through a collaborative grant to the Hällberg and Grünewald groups from the Röntgen Ångström Cluster"



