How glycans soften a virus by stabilizing structure
Scientists from CSSB and their international collaborators reveal key insights into the molecular interactions taking place during the initial steps of Human Papillomavirus (HPV) infection. Their findings were published in the scientific journal Nature Communications.
HPVs are a large family of DNA viruses that cause diseases ranging from infections through warts to throat or genital cancers. While vaccines are available to prevent HPV infection, the development of additional anti-viral strategies is highly desirable as vaccines do not work anymore after the exposure to the virus.
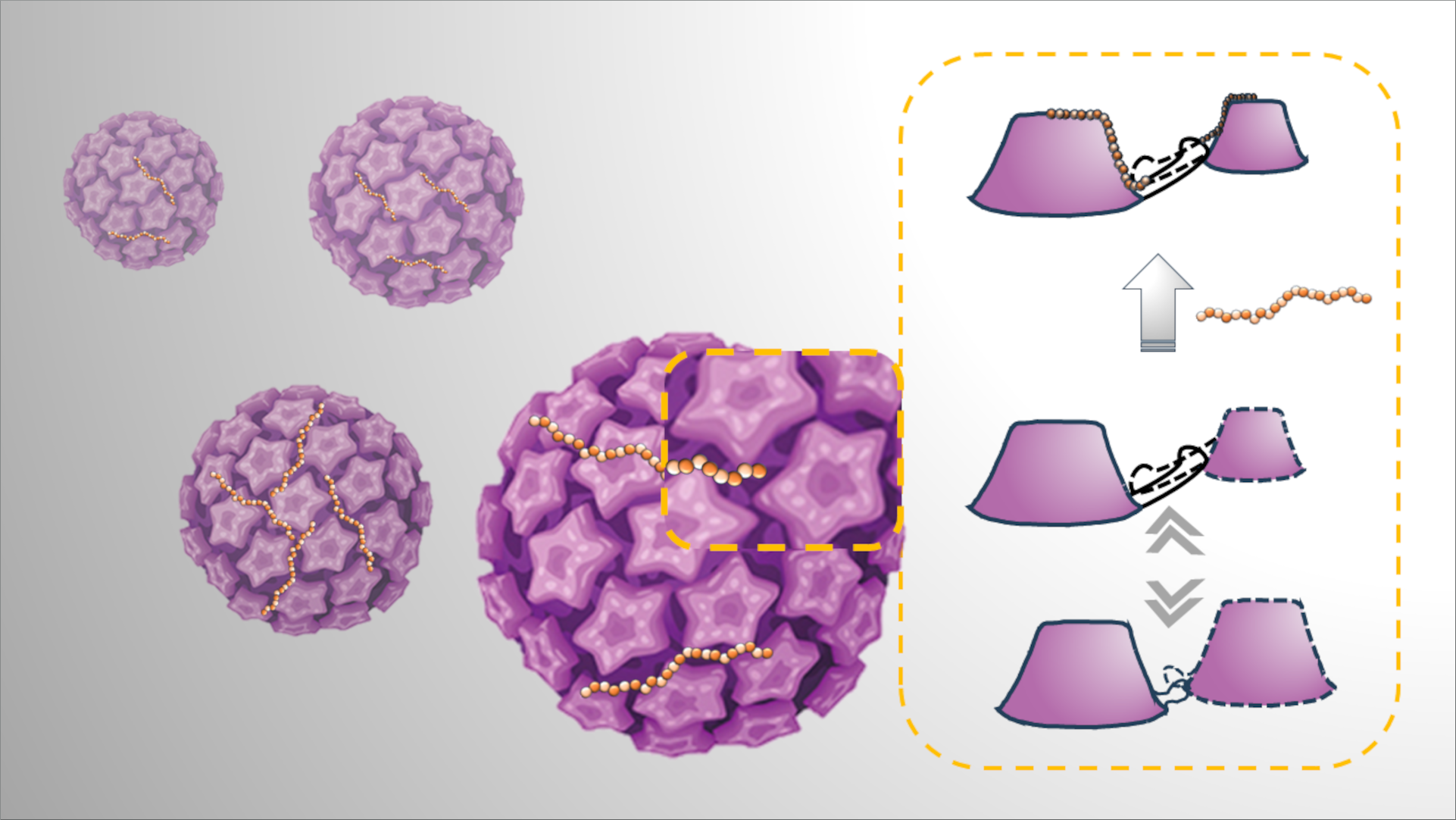
To gain a deeper understanding of the initial interactions between HPV and host basal cells, the researchers in the Uetrecht Group (UzL, DESY) in collaboration with scientists from the University of Groningen and the University of Münster investigated the role played by cellular heparan sulphates (HS) in facilitating HVP entry. Employing a combination of virological assays, hydrogen/deuterium exchange mass spectrometry and atomic force microscopy the researchers determined the effect of viral capsid-HS bonding and structural activation.
HPV capsids initially bind to cells via HS, which are linear polysaccharides present on the cellular surface. “We understood that the interaction between HPVs and HS results in conformational changes in the virus capsid, that are crucial for infection” notes one of the studies first authors Dominik van Bodegraven from the University of Münster “however, these interactions were ill-defined and their molecular nature as well as their specific function remained unclear.”
The researchers used an atomic force microscope (AFM) to image HS-bound HPVs at the single virus particle level and to measure viral mechanical properties such as the viral spring constant, a measure of a virus’ stiffness. “We show that the engagement of several binding sites by a minimum length of HS polysaccharide is needed for structural activation,” explains first author Yuzhen Feng from University of Groningen.
Further investigation of the interaction via hydrogen/deuterium exchange mass spectrometry, which maps local changes of structure and dynamics in proteins, revealed that the binding of HS to HPV particles imparts a reversible force on intercapsomere linkers. “This force stabilizes the otherwise very dynamic viral capsid in an extended conformation,” explains first author Alan Kádek, a former member of CSSB’s Uetrecht group and now at the Institute of Microbiology of the Czech Academy of Sciences, “The capsid is therefore both enlarged and softened which ultimately facilitates viral entry into the host cell.”
Dominik van Bodegraven from the University of Münster adds “Together with our data from experiments on living cells, we can now propose a model showing how these structural and physical changes to the capsid contribute to infection’’.
“Overall, this work reveals new mechanistic insights into the initial steps of HPV infection that will likely advance fundamental science, and moreover may help further the design of novel small compound inhibitors to help combat HPV infections” notes Charlotte Uetrecht, CSSB Group leader and one of the study’s corresponding authors.
Original Publication:
Feng, Y., van Bodegraven, D., Kádek, A. et al. Glycan-induced structural activation softens the human papillomavirus capsid for entry through reduction of intercapsomere flexibility. Nat Commun 15, 10076 (2024). https://doi.org/10.1038/s41467-024-54373-0
German Version:
https://www.uni-luebeck.de/forschung/aktuelles-zur-forschung/aktuelles-zur-forschung/artikel/neue-erkenntnisse-ueber-hpv-infektionen.html