Key Mechanism of Herpesvirus Egress Uncovered
New Potential Target for Antiviral Therapies
An international team of researchers, including CSSB’s Grünewald group (LIV, UHH), Bosse group (MHH) and the cryo-EM multi-user facility, has uncovered crucial details on how herpesviruses exit the cell nucleus without compromising the integrity of the nuclear envelope. The study, published yesterday in the renowned journal Nature Microbiology, utilized advanced electron cryo-tomography to visualize the structures involved. These findings could pave the way for developing more effective antiviral therapies to combat herpesvirus infections.
Herpesvirus infections are widespread globally and cause significant health burdens and life-threatening complications in immunocompromised people.
The study focuses on Herpes simplex virus 1 – the causative agent of oral cold sores – and Pseudorabies virus (PrV), a model virus for human herpes infections. The researchers identified various structures of the herpesviral nuclear egress complex (NEC) protein coat located on the inner nuclear membrane. These structures are critical for the release of capsids into the perinuclear space and their subsequent transfer to the cytosol.
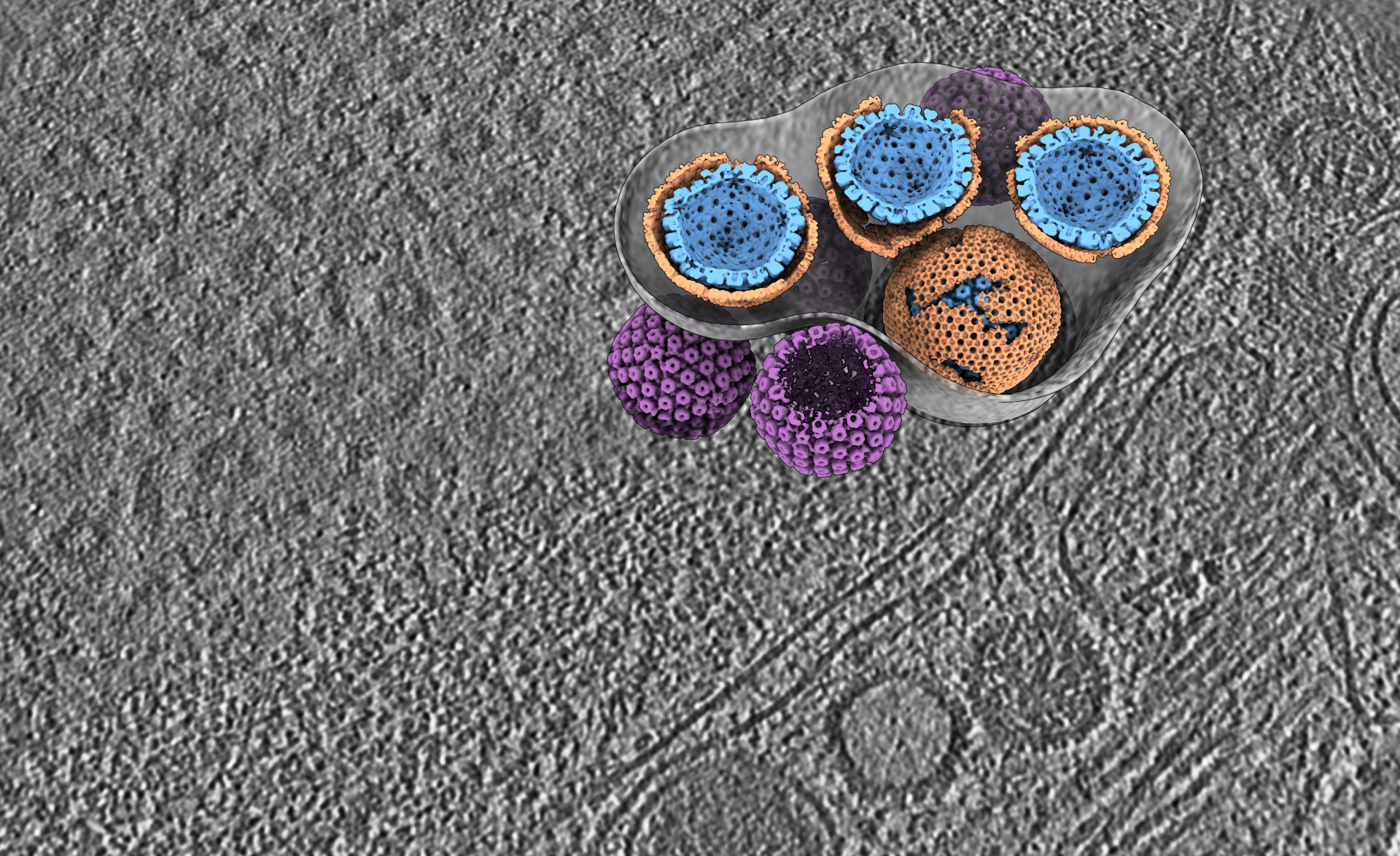
The researchers used the advanced imaging techniques available at CSSB’s cryo-EM multi-user facility, such as electron cryo-tomography, to structurally characterize the interface between the NEC and the transported virus capsid surface in infected cells. These nanoscale insights reveal that the NEC plays a key role in shuttling herpesvirus capsids – the icosahedral protein cages that contain the herpesviral DNA genome – from the nucleus without damaging the nuclear envelope. The findings indicate a remarkable structural flexibility of the NEC, suggesting that the mechanism is not rigid but adaptive.
Dr. Vojtěch Pražák, one of the study's lead authors illustrates: “How do you pass a ball through a double glazed window without breaking it? We can't do it, but herpesviruses figured out how to do the equivalent - to go through the nuclear membranes without rupturing them. This is a really useful skill for them since a damaged nucleus would tell the immune system something is wrong.”
“Our work shows that the the NEC coat formation happens in distinct steps. We were also surprised to the extent the individual NEC components can flex to form different 3D structures, rather than a single uniform lattice,” explains Yuliia Mironova, another lead author of the study, “The detailed characterization of these processes may open new avenues for targeted disruption of virus replication.”
Previous studies have highlighted the importance of the NEC for the viral life cycle, but this study provides the first detailed structural analysis of the NEC-virus particle interfaces in the cellular environment. Prof. Dr. Kay Grünewald, CSSB Deputy Scientific Director and Head of the LIV department Structural Cell Biology of Viruses, emphasizes, “By charting the flexibility of the lateral interactions between proteins in NEC lattices of different curvature directly inside cells, we unveiled the mechanism of curvature induction. Unexpectedly, we further show that capsid to NEC interaction is not restricted to specific capsid positions.”
Overall, the new insights from this study offer promising perspectives for combating herpesvirus infections. The nanoscale structural insights determined by the researchers lay the foundation for understanding the complex nuclear egress mechanism common to all herpesviruses. Accordingly, the results are also relevant for other human-pathogenic herpesviruses, thus providing exciting starting points for the development of antiviral interventions.
Background information
Herpesviruses replicate their DNA within the nucleus of infected cells. After replication, the genome is packed into newly assembled virus capsids, i.e. icosahedral protein containers of about 125 nm diameter. These then exit the nucleus to reach the cytosol of the cell to undergo final assembly and envelopment and are eventually released from the cell. Released virus particles can invade neighboring cells, breaching the cell membrane by membrane fusion. Besides epithelial cells also neurons are infected. In the latter, herpesviruses can establish lifelong persistent infections, termed latency. During latency, no infectious virus is produced from infected cell. Upon stress or other triggers like UV-light infections can reactivate.
The Pseudorabies virus (PrV) was used in the study as a model virus to investigate the nuclear egress mechanisms of herpesviruses. Herpesviruses share many fundamental mechanisms and structures, so insights gained with PrV are often transferable to other herpesviruses. This includes human-pathogenic herpesviruses such as the herpes simplex viruses 1 (HSV-1, here studied as well) and 2 (HSV-2), which can cause cold sores and genital herpes, respectively, and the varicella-zoster virus (VZV), which causes chickenpox and shingles.
Currently, there are only few types of antiviral medications available to treat herpesvirus infections. These can alleviate symptoms and shorten the duration of the infection but are unable to completely eliminate the virus from the body. Therefore, a need for new therapies remains to more effectively combat resistance against the available set of antivirals. This is not only relevant to improve the quality of life for any herpesvirus affected persons but particularly important for immunocompromised individuals, like after organ transplants.
Original Publication:
Pražák, V., Mironova, Y., Vasishtan, D. et al. Molecular plasticity of herpesvirus nuclear egress analysed in situ. Nat Microbiol (2024). https://doi.org/10.1038/s41564-024-01716-8
https://rdcu.be/dLP3D



