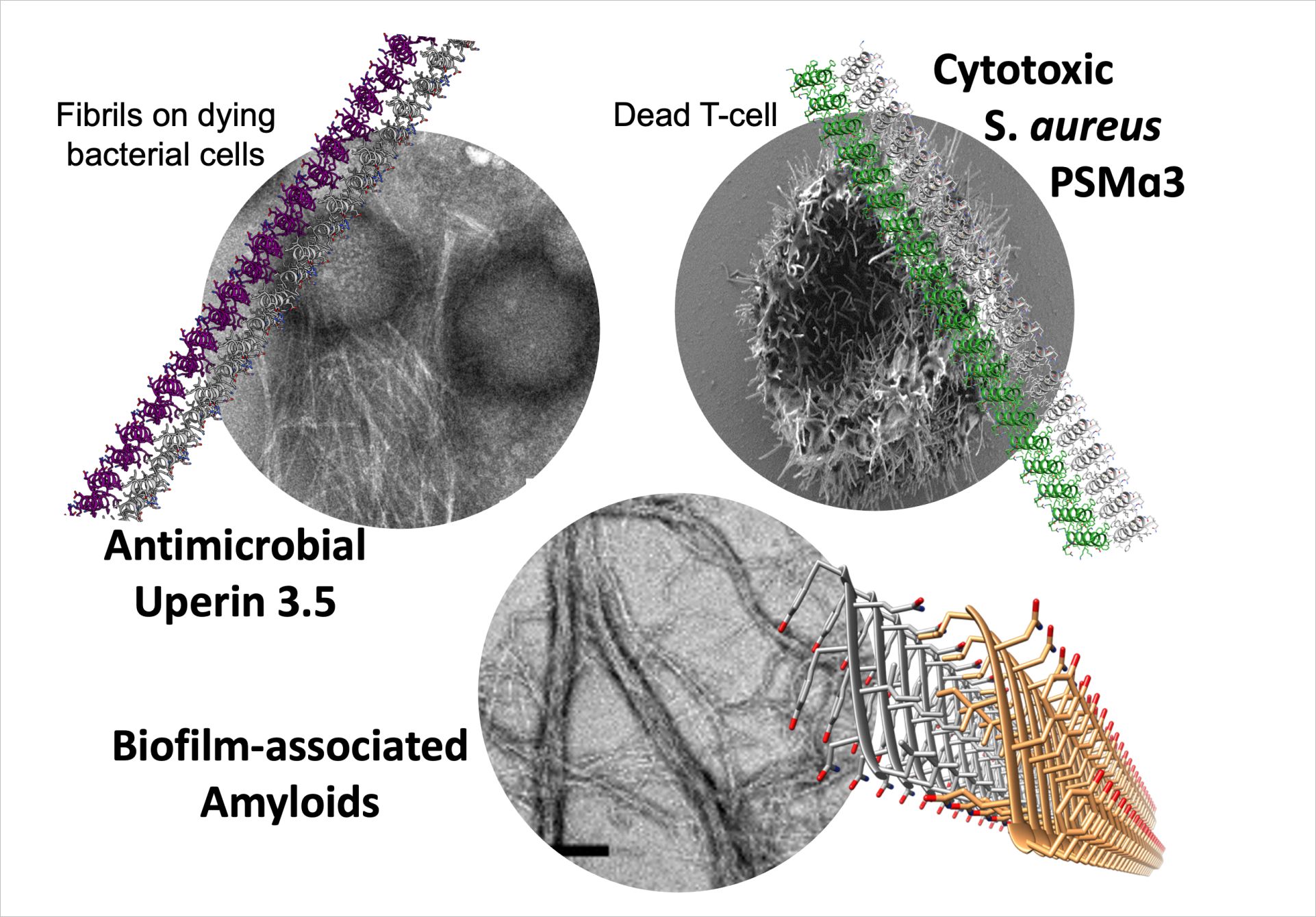
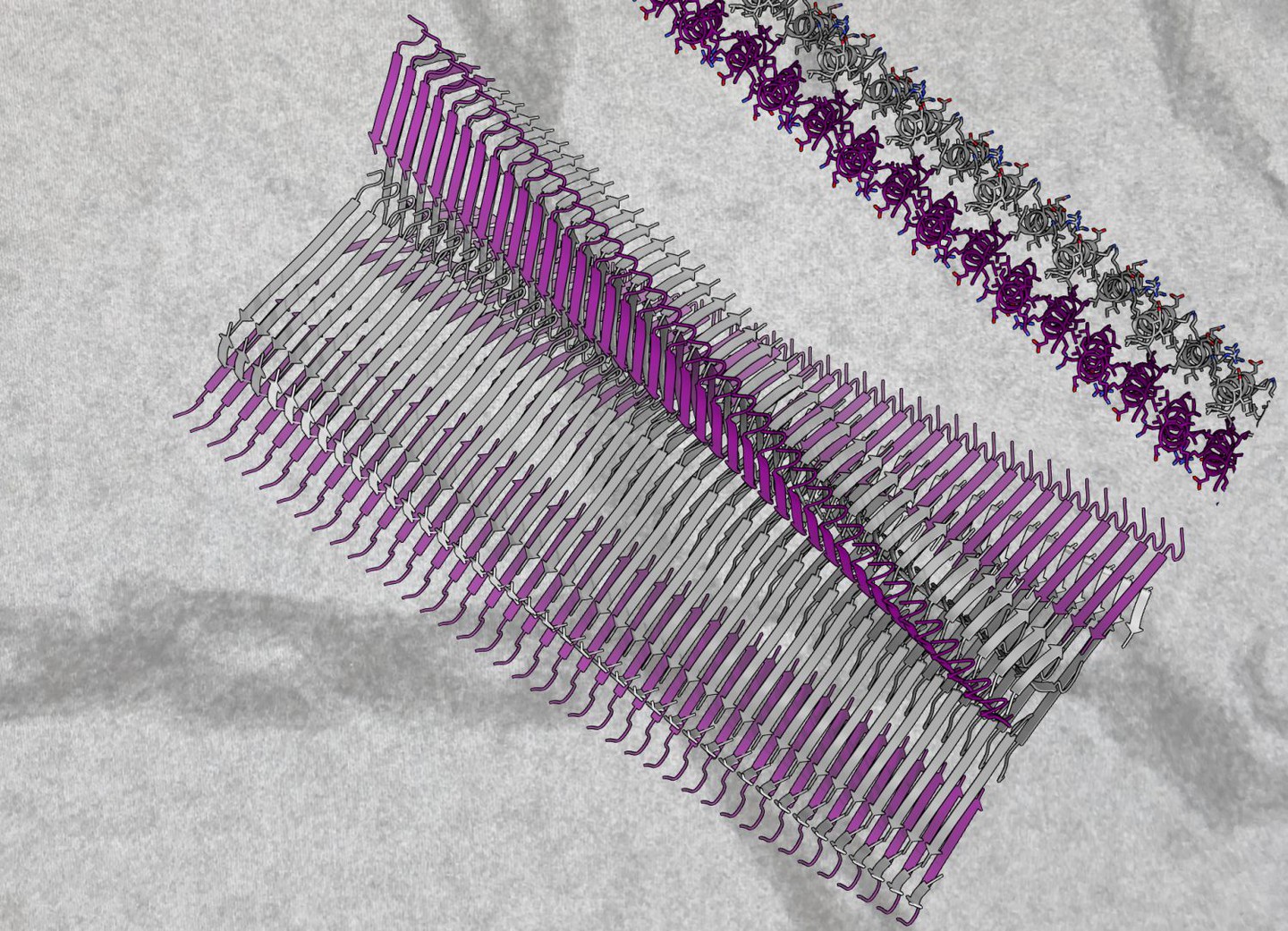
Publications
Preprints
Strati F, Pigozzi Cal Mi, Bloch Y, Mostafavi S, Monistrol J, Golubev A, Rayan B, Gustavsson E, Landau M (2025) Structural and Functional Versatility of the Amyloidogenic Antimicrobial Peptide Citropin 1.3. bioRxiv 2025.01.31.635854; doi: https://doi.org/10.1101/2025.01.31.635854
Rayan B, Barnea E, Indig R, Pantoja CF, Gayk J, Lupu- Haber Y, Upcher A, Argoetti A, Aunstrup Larsen J, Buell AK, Zweckstetter M, Landau M (2025) RNA Selectively Modulates Activity of Virulent Amyloid PSMα3 and Host Defense LL-37 via Phase Separation and Aggregation Dynamics. bioRxiv 2025.06.17.660072; doi: https://doi.org/10.1101/2025.06.17.660072
M. Rumpret, H.J. von Richthofen, M. van der Linden, G.H.A. Westerlaken, C.T. Ormeño, J.A.G. van Strijp, M. Landau, H. Ovaa, N.M. van Sorge, and L. Meyaard. Signal inhibitory receptor on leukocytes-1 recognizes bacterial and endogenous amphipathic α-helical peptides. FASEB J. 35:e21875; 2021.
M. Mayer, Y. Matiuhin, M. Nawatha, O. Tabachnikov, I. Fish, N. Schutz, H. Dvir, M. Landau Structural and Functional Insights into the Biofilm-Associated BceF Tyrosine Kinase Domain from Burkholderia cepacia. Biomolecules. 11, 1196; 2021 doi
R. Malishev, N. Salinas, J. Gibson, A.B Eden, J. Mieres-Perez, Y. Ruiz-Blanco, O. Malka, S. Kolusheva, F-G Klärner, T. Schrader, E. Sanchez-Garcia, C. Wang, M. Landau, G. Bitan, and R. Jelinek. Inhibition of Staphylococcus aureus Biofilm-forming Functional Amyloid by Molecular Tweezers. Cell Chemical Biology S2451-9456(21)00152-5; 2021
P. Ragonis-Bachar and M. Landau. Functional and pathological amyloid structures in the eyes of 2020 cryo-EM. Curr Opin Struct Biol. 22;68:184-193; 2021
2020
N. Salinas, TL. Povolotsky, M. Landau, I. and Kolodkin-Gal. Emerging Roles of Functional Bacterial Amyloids in Gene Regulation, Toxicity, and Immunomodulation. Microbiol Mol Biol Rev 85(1):e00062-20; 2020. Full Text.
Y. Engelberg and M. Landau. The Human LL-37(17-29) Antimicrobial Peptide Reveals a Functional Supramolecular Structure. Nat Commun 11, 3894; 2020 Full Text.
E. Tayeb-Fligelman, N. Salinas O. Tabachnikov, and M. Landau. Determinants of Staphylococcus aureus PSMα3 Cross-α Fibrils Toxicity. Structure 3;28(3):301-313; 2020. Full Text.
2019
S. Perov, O. Lidor, N. Salinas, N. Golan, E. Tayeb-Fligelman, M. Deshmukh, D. Willbold, and M. Landau. Structural Insights into Curli CsgA Cross-β Fibril Architecture Inspired Repurposing of Anti-amyloid Compounds as Anti-biofilm Agents. PLoS Pathog 15(8): e1007978; 2019. Full text
2018
M. Landau. Mimicking cross-α amyloids. Nature Chemical Biology 14(9):833-834; 2018. Full text
B. Brumshtein, S.R. Esswein, M.R. Sawaya, G. Rosenberg, A.T. Ly, M. Landau, and D.S. Eisenberg. Identification of two principal amyloid-driving segments in variable domains of Ig light chains in systemic light-chain amyloidosis. J. Biol. Chem. 293(51) 19659–19671; 2018
N. Salinas, A. Moshe, J-P. Colletier, and M. Landau. Extreme Amyloid Polymorphism in Staphylococcus aureus Virulent PSMα Peptides. Nature Communications 9(1):3512; 2018. Full text
R. Malishev, E. Tayeb-Fligelman, S. David S, M. Meijler, M. Landau*, and R. Jelinek*. Reciprocal Interactions between Membrane Bilayers and S. aureus PSMα3 Cross-α Amyloid Fibrils Account for Species-Specific Cytotoxicity. J. Mol. Biol. 430(10):1431-1441; 2018
M. Landau. SLC9. In: Choi S. (eds) Encyclopedia of Signaling Molecules. Springer, Cham; 2018. DOI: https://doi.org/10.1007/978-3-319-67199-4_101935
2017
M. Landau. Getting in Charge of β-synuclein Fibrillation J Biol. Chem. 29;292(39):16380-16381; 2017
E. Tayeb-Fligelman, O. Tabachnikov, A. Moshe, O. Goldshimidt-Tran, M.R. Sawaya, N. Coquelle, J.-P. Colletier, M. Landau. The Cytotoxic Staphylococcus aureus PSMα3 Reveals a Cross-α Amyloid-like Fibril. Science 355(6327): 831-833; 2017
E. Ratzon, I. Bloch, M. Nicola, E. Cohen, N. Ruimi, N. Dotan, M. Landau, and M. Gal. A Small Molecule Inhibitor of Bruton’s Tyrosine Kinase Involved in B-Cell Signaling. ACS Omega 2 (8), 4398-4410; 2017
2016
E. Tayeb-Fligelman and M. Landau. X-ray structural study of amyloid-like fibrils of Tau peptides bound to small-molecule ligands. Methods in Molecular Biology .1523: 89-100; 2016
E. Reinstein, A. Gutierrez-Fernandez, S. Tzur, C. Bormans, S. Marcu, E. Tayeb-Fligelman, C. Vinkler, A. Raas-Rothschild, D. Irge, M. Landau, M. Shohat, X.S. Puente, D.M. Behar, C. Lopez-Otın. Congenital dilated cardiomyopathy caused by biallelic mutations in Filamin C. Eur J Hum Genet. 24(12):1792-1796; 2016
Bhonker Y, Abu-Rayyan A, Ushakov K, Amir-Zilberstein L, Shivatzki S, Yizhar-Barnea O, Elkan-Miller T, Tayeb-Fligelman E, Kim SM, Landau M, Kanaan M, Chen P, Matsuzaki F, Sprinzak D, Avraham KB. The GPSM2/LGN GoLoco motifs are essential for hearing. Mamm Genome. 27(1-2):29-46: 2016
A. Moshe, M. Landau and D. Eisenberg. Preparation of Crystalline Samples of Amyloid Fibrils and Oligomers. Methods in Molecular Biology 1345: 201-210; 2016
E. Padan and M. Landau. Sodium-Proton (Na+/H+) Antiporters: Properties and Roles in Health and Disease. Alkali Metal Ions: Their Role for Life. Book Series: Metal Ions in Life Sciences. Volume 16 Pages 391-458. Eds Astrid Sigel, Helmut Sigel, and Roland K. O. Sigel. Springer International Publishing AG, Cham, Switzerland 2016; ISSN: 1559-0836
2015
Reinstein E, Orvin K, Tayeb-Fligelman E, Stiebel-Kalish H, Tzur S, Pimienta AL, Bazak L, Bengal T, Cohen L, Gaton DD, Bormans C, Landau M, Kornowski R, Shohat M, Behar DM. Mutations in TAX1BP3 cause dilated cardiomyopathy with septo-optic dysplasia. Hum Mutat. 36(4):439-42: 2015
2014
B. Brumshtein, SR. Esswein, M. Landau, CM. Ryan, JP. Whitelegge, ML. Phillips, D. Cascio, MR. Sawaya, and DS. Eisenberg. Formation of amyloid fibers by monomeric light chain variable domains. J Biol Chem 289(40):27513-25; 2014
2013
K. Kondapalli , A. Hack , M. Schushan , M. Landau , N. Ben-Tal, and R. Rao. Functional evaluation of autism-associated mutations in NHE9. Nat Commun 4: p. 2510; 2013
N. Gal, A. Morag, S. Kolusheva, R. Winter, M. Landau, R. Jelinek. Lipid Bilayers Significantly Modulate Cross-Fibrillation of Two Distinct Amyloidogenic Peptides. J. Am. Chem. Soc 135(36):13582–13589; 2013.
L. Jiang, C. Liu, D. Leibly, M. Landau, M. Zhao, M. Hughes, and D.Eisenberg. Structure-based discovery of fiber-binding compounds that reduce the cytotoxicity of amyloid beta. eLife 2:e00857; 2013.
2012
R. Loewenthal, N. Rosenberg, R. Kalt, R. Dardik, M. Landau, V. Yahalom, O. Avishai, O. Frenkel, E. Gazit, D.M. Steinberg, S. Lipitz, O. Salomon. Compound heterozygosity of HLA-DRB3*01:01 and HLA-DRB4*01:01 as a potential predictor of fetal neonatal alloimmune thrombocytopenia. Transfusion 53(2):344-52; 2013;2012
R. Mor-Cohen, N. Rosenberg, Y. Einav, E. Zelzion, M. Landau, W. Mansour, Y. Averbukh and U. Seligsohn. Unique disulfide bonds in the epidermal growth factor (EGF) domains of β3 affect the structure and function of αIIbβ3 and αvβ3 integrins in a different manner. J Biol Chem 16;287(12):8879-91; 2012.
A. Laganowsky, C. Lui, M.R. Sawaya, J.P. Whitelegge, J. Park, M. Zhao, A. Pensalfini, A.B. Soriaga, M. Landau, P.K. Teng, D. Cascio, C. Glabe, D. Eisenberg. Atomic View of a Toxic Amyloid Small Oligomer. Science 335(6073):1228-31; 2012.
2011
JP. Colletier*, A. Laganowsky*, M. Landau*, M. Zhao*, A.B. Soriaga*, L. Goldschmidt, D. Cascio, M.R. Sawaya, and D. Eisenberg. Molecular Basis for Amyloid-β Polymorphism. PNAS 108(41):16938-43; 2011. *Authors contributed equally
M. Landau, M.R. Sawaya, K.F. Faull, A. Laganowsky, L. Jiang, S.A. Sievers, J. Liu, J.R. Barrio and D. Eisenberg. Towards a Pharmacophore for Amyloid. PLoS Biology 9(6): e1001080; 2011.
M. Schushan, M. Landau, E. Padan and N. Ben-Tal (2011). Two conflicting NHE1 model-structures: Compatibility with experimental data and implications for the transport mechanism. J. Biol. Chem. 286, le9; 2011.
2010
M. Landau and N. Rosenberg. Molecular Insight into Human Platelet Antigens: Structural and Evolutionary Conservation Analyses offer New Perspective to Immunogenic Disorders. Transfusion 51(3):558-69; 2010.
A. Laganowsky, J.L.P. Benesch M. Landau, L. Ding, M.R. Sawaya, D. Cascio, Q. Huang, C.V. Robinson, J. Horwitz, and D. Eisenberg. Crystal structures of truncated α and αB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Prot. Sci. 19(5):1031-43; 2010.
H. Hauschner, M. Landau, U. Seligsohn, and N. Rosenberg. A unique interaction between αIIb and β3 in the head region is essential for outside-in signaling related functions of αIIbβ3 integrin. Blood 115(22):4542-50; 2010.
J.W. Wiltzius*, M. Landau*, R. Nelson*, M.R. Sawaya*, M.I. Apostol, L. Goldschmidt, A.B. Soriaga, D.Cascio, K. Rajashankar and D. Eisenberg. Molecular mechanisms for protein-encoded inheritance. Nat. Struct. Mol. Biol. 16(9) 973-8; 2009. *Authors contributed equally
2009
M. Zucker, A. Zivelin, M. Landau, N. Rosenberg and U. Seligsohn. Three residues at the interface of factor XI monomers augment covalent dimerization of factor XI. J Thromb Haemost. 7(6):970-5; 2009.
2008
R. Mor-Cohen, N. Rosenberg, M. Landau, J. Lahav and U. Seligsohn. Specific cysteines in β3 are involved in disulfide bond exchange-dependent and -independent activation of αIIbβ3. J Biol Chem. 283(28):19235-44; 2008.
M. Landau and N. Ben-Tal. Dynamic Equilibrium between Multiple Active and Inactive Conformations Explains Regulation and Oncogenic Mutations in ErbB Receptors. Biochim Biophys Acta. Reviews on cancer 1785(1):12-31; 2008.
2007
M. Landau, K. Hertz, E. Padan and N. Ben-Tal. Model structure of the Na+/H+ exchanger 1 (NHE1) of the human heart: functional and clinical implications. J Biol Chem. 282(52):37854-63; 2007.
R. Mor-Cohen, N. Rosenberg, H. Peretz, M. Landau, B.S. Coller, A. Awidi, U. Seligsohn. Disulfide bond disruption by a β3-Cys549Arg mutation in six Jordanian families with Glanzmann thrombasthenia causes diminished production of constitutively active αIIbβ3. J Thromb Haemost. 98(6):1257-65; 2007
C. Bozzao, V. Rimoldi, R. Asselta, M. Landau, R. Ghiotto, M.L. Tenchini, R. De Cristofaro, G. Castaman and S. Duga. A novel factor XI missense mutation (Val371Ile) in the activation loop is responsible for a case of mild type II factor XI deficiency. FEBS J. 274: 6128-6138; 2007.
M. Zucker, A. Zivelin, M. Landau, O. Salomon, G. Kenet, F. Bauduer, M. Samama, J. Conard, H. Denninger, A.B. Hani, M. Berruyer, D. Feinstein, U. Seligsohn. Characterization of seven novel mutations causing FXI deficiency. Haematologica, 92(10):1375-80; 2007.
2006
N. Rosenberg, S. Lalezari, M. Landau, B. Shenkman, U. Seligsohn and S. Izraeli. Trp207Gly in platelet glycoprotein Ibα is a novel mutation which disrupts the connection between the leucine-rich repeat domain and the disulfide loop structure and causes Bernard-Soulier syndrome. J Thromb Haemost 5(2):378-86; 2006.
H. Peretz, N. Rosenberg, M. Landau, S. Usher, E.J.R. Nelson, R. Mor-Cohen, D.L. French, B.W. Mitchell, S.C. Nair, M. Chandy, B.S. Coller, A. Srivastava and U. Seligsohn. Molecular diversity of Glanzmann thrombasthenia in southern India: new insights into mRNA splicing and structure-function correlations of αIIbβ3 integrin (ITGA2B, ITGB3). Human Mutation 27:359-369, 2006.
M. Landau and N. Zisapel. The low affinity binding of melatonin to calmodulin: use of computational methods to explain its physiological relevance. Melatonin: from molecules to therapy, S.R. Pandi-Perumal and D.P. Cardinali. Hauppauge, NY: Nova Science Publishers, Inc.; 2006.
2005
N. Rosenberg, H. Hauschner, H. Peretz, R. Mor-Cohen, M. Landau, B. Shenkman, G. Kenet, B.S. Coller, A. A. Awidi and U. Seligsohn. A 13bp Deletion in αIIb Gene is a Founder Mutation that Predominates in Palestinian Arab Patients with Glanzmann Thrombasthenia. J Thromb Haemost 3: 2764-72, 2005.
M. Landau, I. Mayrose, Y. Rosenberg, F. Glaser, E. Martz, T. Pupko and N. Ben-Tal. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res 33: W299-W302, 2005.
Y. Fromovich-Amit, A. Zivelin, N. Rosenberg, M. Landau, R. Jean-Philippe and U. Seligsohn. Of four mutations in the factor VII gene in Tunisian patients, one novel mutation (Ser339Phe) in three unrelated families abrogates factor X activation. Blood Coagul Fibrinolysis 16: 369-374, 2005.
M. Mark-Danieli, N. Laham, M. Kenan-Eichler, A. Castiel, D. Melamed, M. Landau, N.M. Bouvier, M.J. Evans and E. Bacharach. Single point mutations in the zinc finger motifs of the human immunodeficiency virus type 1 nucleocapsid alter RNA binding specificities of the gag protein and enhance packaging and infectivity. J Virol 79: 7756-67, 2005.
2004
M. Landau, S.J. Fleishman and N. Ben-Tal. A Putative mechanism for down-regulation of the catalytic activity of the EGF receptor via direct contact between its kinase and C-terminal domains. Structure 12: 2265-75, 2004.
Y. Fromovich-Amit, A. Zivelin, N. Rosenberg, H. Tamary, M. Landau and U. Seligsohn. Characterization of mutations causing factor VII deficiency in 61 unrelated Israeli patients. J Thromb Haemost 2:1774-1781, 2004.
A. Zivelin, T. Ogawa, S. Bulvik, M. Landau, J.R. Toomey, J. Lane, U. Seligsohn and D. Gailani. Severe factor XI deficiency caused by a Gly555 to Glu mutation (factor XI-Glu555): a cross-reactive material positive variant defective in factor IX activation. J Thromb Haemost 2: 1782-1789, 2004.
A. Vysokovsky, R. Saxema, M. Landau, A. Zivelin, R. Eskaraev, N. Rosenberg, U. Seligsohn and A. Inbal. Seven novel mutations in Factor XIII A-subunit gene causing hereditary Factor XIII deficiency in 10 unrelated families. J Thromb Haemost 2: 1790-1797, 2004.
O. Ashur-Fabian, A. Avivi, L. Trakhtenbrot, K. Adamsky, M. Cohen♠, G. Kajakaro, A. Joel, N. Amariglio, E. Nevo and G. Rechavi. Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation. Proc Natl Acad Sci USA 101: 12236-12241, 2004.
N. Rosenberg, M. Landau, J. Luboshitz, G. Rechavi and U. Seligsohn. A novel PHE171CYS mutation in integrin αIIb causes Glanzmann Thrombasthenia by abrogating αIIbβ3 complex formation. J Thromb Haemost 2: 1167-1175, 2004.
♠ My maiden name.