CSSB Scientists Reveal High Resolution Injectisome
The Marlovits group, at the Centre for Structural Systems Biology CSSB, has revealed the first high resolution structure of an active, substrate-engaged bacterial type-III secretion system. The research study, published in Nature Communications, provides molecular insights into the structure and function of the translocation process which is fundamental to the virulence of many pathogenic bacteria. According to the researchers, visualizing this process at the molecular level is essential for our understanding of host-pathogen biology and the development of novel therapies targeting bacterial infection.
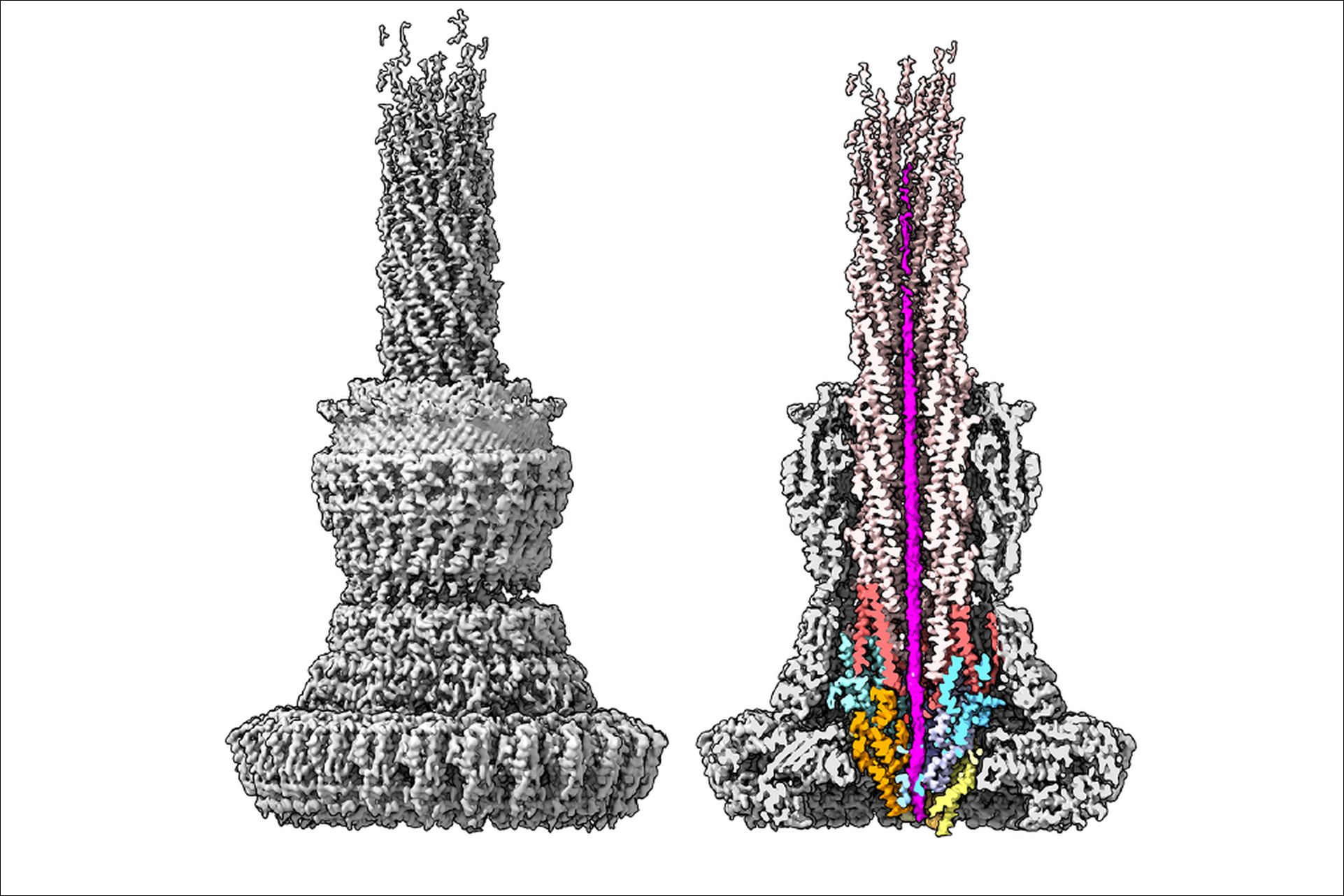
Structure of the type III secretion system of Salmonella, imaged with the cryo-electron microscope. The section through the complex (right), shows in magenta a bacterial toxin protein during transport through the narrow needle cannula. For successful transport, toxin proteins must unfold completely and take on a new shape. The proteins that form the export apparatus and the needle filament secretion channel are also stained. The researchers were able to observe a bacterial protein inside the needle complex for the first time using the high-resolution cryo-electron microscope at CSSB. IMAGE: Thomas C Marlovits
To establish infection in human hosts, several Gram-negative bacteria such as Salmonella or E. coli, rely on type III secretion systems also known as injectisomes. The injectisome facilitates translocation, a process during which bacterial effector proteins travel through a needle-like structure and are injected directly into the human host cell. The proteins then help the pathogen to invade host tissue and propagate infection.
The Marlovits group has been investigating the molecular mechanisms of the injectisome’s needle complex, a continuous conduit for effector protein translocation, for several years. “The injectisome is an extremely complex yet fascinating molecular machine,” explains group leader Thomas Marlovits “A recent biochemistry breakthrough combined with the resolution revolution in single particle electron cryo-microscopy enabled us to generate this first high-resolution snapshot of the needle complex in action.”
Occurring at rate of 7-60 molecules per second, the rapid secretion of molecules through the needle complex is difficult to visualize. To overcome this challenge Sean Miletic utilized a biochemical trick to trap the effector protein SptP within in the needle complex. “Fusing a biochemical tag to the C-terminal of SptP prevents the protein from secreting through the needle complex,” explains Miletic. This method to plug the secretion conduit was established previously by research groups at the CSSB to study protein secretion by the system1,2.
With SptP trapped inside the needle complex, the researchers could effectively examine this active state using microscopes available at CSSB’s multi-user cryo-EM facility. “Surprisingly, our initial images revealed only subtle differences between the needle complex with SptP inside and an empty needle complex,” notes Jiri Wald who collected the cryo-EM images. To capture these subtle movements of the needle complex, the researchers turned to single particle cryo electron microscopy which generates high-resolution images of single particles or complexes.
The cryo-EM data confirmed the essential role of the needle complex export apparatus (EA) which functions as the needle complex’s entry portal. “We determined that the EA forms a three-point interface with the bacterial protein SptP,” explains Dirk Fahrenkamp who built the atomic model “The EA channel contains two hydrophilic Q-belts that sandwich a hydrophobic M-gate and a movable SpaR lid. Together these elements function as both a gate and guide, to engage and steer bacterial proteins towards the tip of the needle.” Understanding and visualizing the molecular mechanisms employed by bacteria to invade human cells at atomic resolution is essential for the development novel therapies which are able to fight infection in a targeted and intelligent manner. Nikolaus Goessweiner-Mohr, who also contributed to the paper, adds “What renders these achievements borderline magic, is the immense speed and precision with which data collection, processing and interpretation took place last year”.
“While these results enrich our understanding of the type three secretion system, this study is also notable in that it was truly a group effort,” explains Marlovits “not only did each of the paper’s four first authors* contribute their own vital expertise but the collaboration efforts of the cryo-EM facility and the support of the CSSB media kitchen were essential to the success of this study.”
The Centre for Structural Biology (CSSB), a joint initiative of three universities and six research institutes, investigates the molecular mechanisms of host pathogen interactions. The Marlovits group is one of thirteen research groups at CSSB in Hamburg, Germany. Thomas Marlovits is a Deputy Scientific Director and Senior Scientist at CSSB. He is also a professor and director of Institute of Structural and Systems Biology at University Medical Center Hamburg-Eppendorf and is affiliated with DESY - German Electron Synchrotron Centre.
Source:
Miletic S, Fahrenkamp D, Goessweiner-Mohr N, Wald J, Pantel M, Vesper O, Kotov V, Marlovits TC (2021) Substrate-engaged type III secretion system structures reveal gating mechanism for unfolded protein translocation. Nature Communications
DESY News Story:
https://www.desy.de/news/news_search/index_eng.html?openDirectAnchor=2036&two_columns=0
References:
1. Radics J, Königsmaier L, Marlovits TC (2014) Structure of a pathogenic type 3 secretion system in action. Nat. Struct. Mol. Biol. 21, 82–87
2. Dohlich K, Zumsteg AB, Goosmann C (2014) Kolbe M A substrate-fusion protein is trapped inside the type III secretion system channel in Shigella flexneri. PLoS Pathog 10, e1003881



