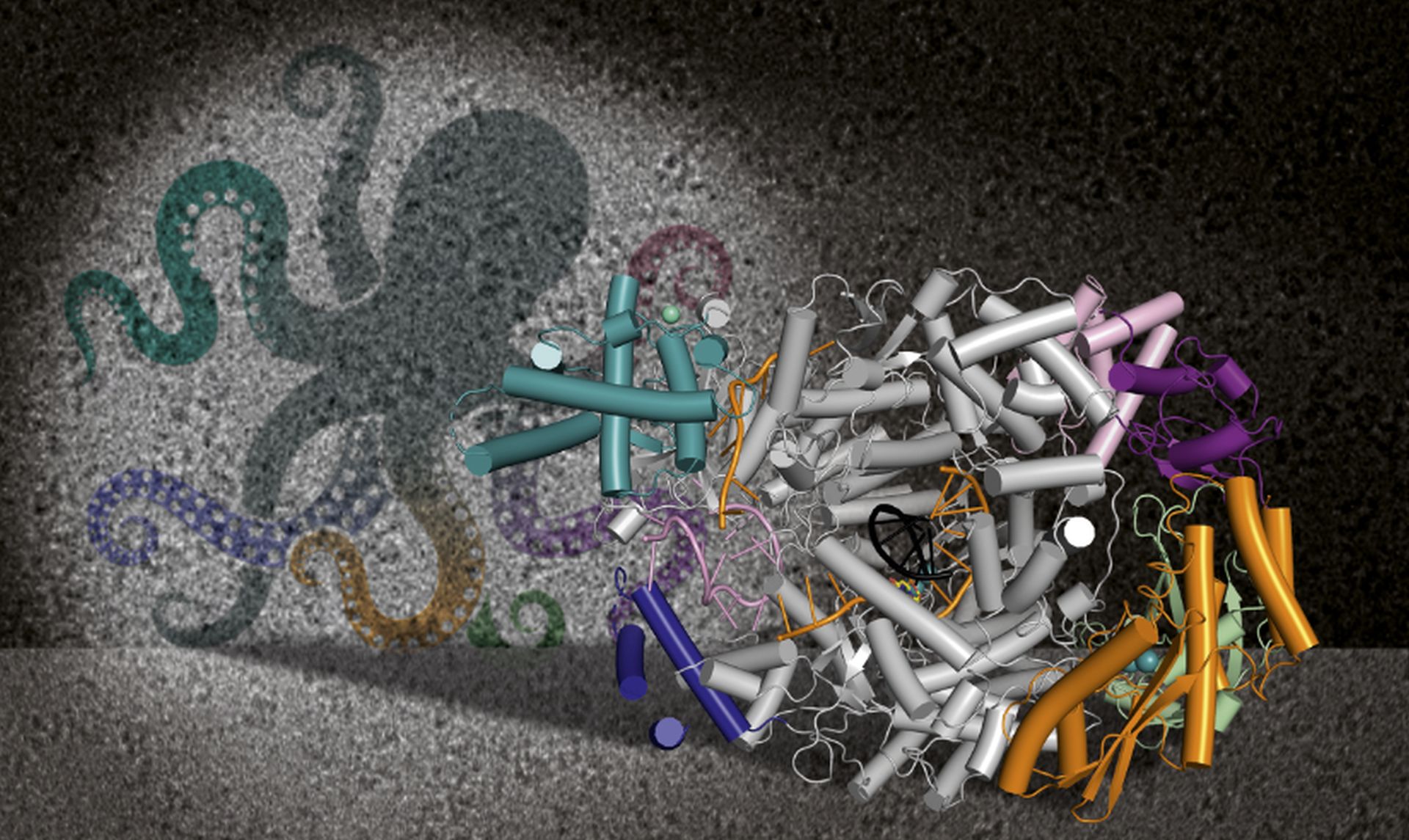
Wrangling an octopus-like viral replication machine
Researchers from CSSB's Grünewald group (HPI/ UHH) in collaboration with research groups from the Bernhard Nocht Institute for Tropical Medicine (BNITM) and EMBL Grenoble have studied nine structures of an essential Lassa virus protein in different functional states. The protein is necessary for virus replication and thus provides excellent targets for antiviral agents. The results have now been published in the journal Nature Communications.
Endemic in Western African countries, Lassa virus is transmitted to humans through food or household items that are contaminated with the urine or faeces of Mastomys rats. Even though many people who become infected with Lassa virus are asymptomatic, one in five infections results in severe haemorrhagic disease, attacking vital organs like the liver, spleen, and kidneys. The World Health Organization (WHO) lists Lassa fever as a significant public health threat. The infectious disease has a high epidemic potential; without a vaccine or reliable and effective medications.
"Although research groups worldwide are working on a vaccine, an effective antiviral drug is urgently needed to reduce the number of severe cases and deaths," explains Dr. Maria Rosenthal, BMBF junior research group leader at BNITM. This is where structural biology can help.
Promising Lassa virus targets for science.
The collaboration between research groups at EMBL Grenoble, BNITM, and Kay Grünewald's (HPI/UHH) lab at CSSB resulted in a paper recently published in Nature Communications. It details nine structures of the Lassa viral polymerase in different functional configurations. In an infected cell, this enzyme replicates the virus’s genetic information and ensures the production of building blocks for the construction of new viruses - it is therefore essential for the survival of the virus.
The polymerase has a peculiar architecture with a core and flexible outer components. “We call the Lassa virus polymerase an ‘octopus’ because it’s like it has arms floating around, which makes it very hard to catch in one position,” said Tomas Kouba, a postdoc in the Cusack Group and one of the three lead authors of the paper.
‘When you observe this ‘octopus’, you also want to see how it behaves, how it moves its arms to eat or catch something,” explained Kouba. "However, these movements are very fast, which makes them difficult to visualize with sufficient precision."
To further investigate the polymerase, the international research group used the modern technique of cryo-electron microscopy: “The power of cryo-EM is that you take millions of pictures of the protein. Then you can sort them into several boxes: one box with an arm up, and then one box with the arm down,” Rosenthal added. “In the end, you have more highly resolved images of octopus arms in a variety of positions, so you can model them.”
These studies provide crucial insights into the Lassa virus, which has only four different viral proteins ‒ very few compared to other viruses, such as herpes or SARS-CoV-2, which have dozens of components. The proteins make up for their small number by assuming multiple functions simultaneously, like a Swiss Army knife. The researchers could, for example, visualize polymerase activity and propose how to block viral replication.
Teamwork for success
To achieve this scientific success, BNITM joined forces with the group of Dr. Stephen Cusack, head of EMBL in Grenoble. “Stephen Cusack was at the forefront of research on influenza and ahead of the field with respect to bunyaviruses and arenaviruses,” said Prof. Stephan Günther, the head of the virology department at BNITM. “We thought it was excellent synergy because he already had a lot of technological knowledge we could transfer to the Lassa polymerase.”
Lassa polymerase has properties that make it difficult to determine its structure, which junior research group leader Rosenthal had been trying to do for several years. "To be able to study the protein, we first had to produce it in sufficient quantities. After long trials with different production systems, I went to Grenoble to test the system used by the Cusack group for influenza polymerase, and it worked excellently for the Lassa polymerase," Rosenthal said.
Building on this success, further research funding for the collaborative excellence project was obtained from the Leibniz Association together with Prof. Kay Grünewald.
Macromolecular crystallography ‒ an imaging technique commonly used in structural biology ‒ couldn’t be used to observe the Lassa polymerase, because the protein moved too much to capture all the arms. Cryo-electron microscopy (cryo-EM) offered the solution as it enables snapshots of the protein to be taken in different functional states. "We applied to Lassa the techniques that Kouba and Joanna Wandzik [a former EMBL PhD student] developed to visualise influenza polymerase in action," Cusack explained. " We used cryo-EM facilities both in Grenoble and at the Centre for Structural Systems Biology.”
The researchers complemented their findings on the structure of Lassa polymerase with further experiments on the regulation of enzyme function and the importance of individual components of the enzyme. "Only through the combined expertise of all partners was it possible to visualize and understand this complex enzyme," Rosenthal emphasizes.
One Health: preparedness
The collaboration also explores other segmented negative-strand RNA viruses on WHO’s priority list. This includes pathogens causing certain insect-borne viral diseases whose geographical spread may be affected by climate change.
Formerly located mostly in tropical areas, these viruses are extending their range. This is already the case for tick-borne Crimean-Congo haemorrhagic fever virus, which has spread into southern Europe, or mosquito-borne Rift Valley fever, which is expected to spread.
Even though these viruses differ significantly in many respects, their polymerases are closely related, and identifying similarities between them could help develop a broad-spectrum antiviral to tackle them all.
“Structural biology is important to understand what we are actually looking at,” Rosenthal said. “How could you win a fight if you don’t really know who you’re fighting? If we know how these viruses work, then we have more ideas on how to tackle them.”
Original Publication:
Kouba T., Vogel D., Thorkelsson S.R, et al.; Conformational changes in Lassa virus L protein associated with promoted binding and RNA synthesis activity, Nature Communications, 2 December 2021;
DOI 10.1038/s41467-021-27305
Original News Story:
https://www.embl.org/news/science/wrangling-an-octopus-like-viral-replication-machine/
German News Story:
https://www.bnitm.de/aktuelles/mitteilungen/7743-mechanismus-einer-krakenartigen-virus-vermehrungsmaschinerie-entschluesselt/



